Abstract
Background: In the context of a rising trend in labor induction cases, limited research has explored the efficacy of the simultaneous use of misoprostol and Foley catheter methods. This clinical trial investigates the efficacy and safety of combining these labor induction techniques compared to the use of oral misoprostol alone.
Methods: This randomized, open-label clinical trial was conducted on pregnant women candidates for induction of labor due to various medical indications referred to Shariati Hospital, Tehran. The oral misoprostol plus Foley catheter group received 50 mg of oral misoprostol every 4 hours, along with the insertion of a Foley catheter into the cervix under sterile conditions. The comparison group received only oral misoprostol. The Bishop scores in this study were measured prior to induction, and at 6 hours and 12 hours after the initiation of the intervention by a single specialist. Statistical comparisons included the number of deliveries within 24 hours, Bishop score, oxytocin dosage, and maternal and fetal complications.
Results: The two groups were homogeneous in regard to age (27.11 ± 3.88 vs. 26.46 ± 4.95, P=0.26), gestational week (P = 0.44), and BMI (P = 0.88). The combination of the Foley catheter and oral misoprostol group showed significantly higher Bishop scores at 6 hours (P < 0.001) and 12 hours (P = 0.02). The oral misoprostol alone group exhibited a significantly higher rate of cases failing to deliver within 24 hours of induction compared to the combination treatment group (73.4% vs. 11.6%, P < 0.001). Furthermore, the oral misoprostol alone group demonstrated significantly higher incidences of adverse outcomes, including uterine tachysystole (17.6% vs. 3.85%, P < 0.001), NICU hospitalization (8% vs. 1.54%, P = 0.015), abnormal Apgar score at five minutes (4.8% vs. 0%, P=0.01), and meconium presence (7.2% vs. 1.54%, P = 0.03).
Conclusion: This study suggests that the combined method for labor induction is more appropriate due to its quicker and more impactful results, along with lower complication rates.
Introduction
Induction of labor involves the initiation of uterine contractions to facilitate the completion of labor before spontaneous contractions begin. In recent years, there has been a significant surge in the frequency of induced labor, with rates in the United States escalating from 9.5% in 1990 to 27.1% in 20181. Indications for induction include post-term pregnancy, premature rupture of membranes (PROM), conditions associated with high blood pressure like pre-eclampsia and eclampsia, diabetes, chorioamnionitis, and pregnancies involving twins, with post-term pregnancy being one of the most common indications2.
Despite its widespread use, induction of labor carries certain risks, including an elevated risk of infection, hyperstimulation, fetal distress, uterine rupture, and an increased risk of cesarean section3, 4, 5. For women with an unfavorable cervix, the induction process often begins with cervical ripening. Various methods exist for inducing labor and ripening, categorized into non-drug methods such as amniotomy and Foley catheter, and drug methods such as the use of prostaglandins. Prostaglandins stand out as one of the most extensively employed pharmaceuticals for labor induction, particularly in regions with favorable conditions. They can be administered orally, intravenously, or topically in the vagina or cervix6. Previous studies have demonstrated that misoprostol (PGE1) is more effective than prostaglandins E2 (PGE2) and oxytocin7.
Due to the lower incidence of uterine hyperstimulation and fetal complications, prescribing misoprostol in oral form is preferred over vaginal administration. Its availability and cost-effectiveness are among other significant advantages of misoprostol8. The incidence of side effects with misoprostol varies depending on the method of administration, ranging from 8% (vaginal method) to 22% (oral method) and 34% (sublingual method). It proves effective in regions with more limited resources. The purpose of using the Foley catheter is to prepare the cervix (ripening) through mechanical dilation of the cervical canal and an increase in the endogenous secretion of prostaglandins9. Previous studies have consistently reported equal efficacy and fewer occurrences of hyperstimulation, uterine, and fetal complications following the use of the Foley catheter compared to other methods of labor induction10, 11. It appears that the use of the Foley catheter does not elevate the risk of infection12.
The optimal method for inducing labor is currently a topic lacking consensus. Considering the distinct mechanisms of action for each of the pharmacological and mechanical methods of labor induction, there is potential for a synergistic effect when these methods are used in combination. However, concerns exist regarding potential adverse effects on both the mother and the neonate13, 14. Due to limited treatment options and relatively few studies addressing the efficacy and safety of simultaneous use, especially with the oral form of misoprostol, there is a need for further investigation13. Therefore, we have decided to conduct a clinical trial study to assess the effectiveness and side effects of combining these two labor induction methods. This study aims to compare the outcomes with the use of oral misoprostol alone in a group of Iranian women with different indications.
Methods
The present study is a clinical trial conducted on pregnant women with a gestational age of 37 weeks or more who were candidates for induction of labor due to various medical indications. The trial compared the effectiveness and safety of two methods of labor induction: the combination of mechanical (Foley catheter) and pharmacological (misoprostol) methods versus the use of misoprostol alone. The study was conducted in the Obstetrics and Gynecology Department of Shariati Hospital in Tehran during the first three months of 2022.
Sample size determination was based on the results of a study by Hossein et al. 13, where the rates of failed induction within the first 24 hours were 11.8% for the combination of oral misoprostol plus Foley catheter group and 28.7% for the oral misoprostol group. Considering an 85% power of the test, a type I error level of 0.05, and an estimated attrition rate of 15%, the sample size for each study arm was calculated to be 132 participants.
The inclusion criteria were as follows: the need for labor induction as determined by a physician, maternal age of 18 years or older, gestational age of 37 weeks or more, singleton pregnancy, and a Bishop score of less than 6. Women with contraindications to labor induction, such as cephalopelvic disproportion, placenta previa, previous uterine surgery, multiple pregnancies, and medical conditions contraindicating vaginal delivery, were excluded from the study.
In this study, eligible women were randomly allocated to two groups: one receiving oral misoprostol and the other receiving a combination of oral misoprostol and a Foley catheter, following detailed explanations about the study procedures and obtaining written consent from the participants. It's important to note that blinding was not implemented due to the nature of the study design.
Upon entry into the study, eligible participants underwent a comprehensive clinical examination conducted by a gynecologist and their assistant. All relevant demographic information, medical history, details of the pregnancy, and cervical scores were thoroughly documented in specialized forms.
The pregnant women in the combination of oral misoprostol with Foley catheter group were administered oral misoprostol tablets at a dosage of 50 mg every 4 hours, with a maximum of three doses a day. Prior to each dose, the fetal and uterine conditions were assessed, and upon confirmation, the specialist doctor would prescribe the next dose. Subsequently, a sterile Foley catheter of suitable size was inserted into the cervix and secured after evaluating the condition of the cervix and the fetus. The Foley catheter balloon was filled with 30 ml of distilled water or normal saline and fixed to the patient's thigh. In the control group, patients received only the prescribed misoprostol pill, following the same procedure. The Bishop scores in this study were measured prior to induction, and 6 and 12 hours after the initiation of the intervention by a single specialist, which helps to maintain consistency and reduce variability in the scoring process.
If labor did not commence within 24 hours after the intervention in both groups, the induction process was deemed unsuccessful. The decision to continue the treatment process depended on the specialist doctor's opinion. Various parameters, including the number of childbirths within 24 hours after the intervention, the time interval from the beginning of the intervention to delivery, the frequency of cesarean sections, cases of no change in the cervix in the first 24 hours, the dose of oxytocin received, and maternal and fetal complications (such as uterine systolic tachycardia, hypertonic uterine contractions, uterine rupture, postpartum hemorrhage, infection, asphyxia, Apgar score less than 7), and NICU hospitalization, were statistically compared between both groups.
In the statistical analysis, a t-test was employed to compare the time interval between induction and delivery across the two groups. Additionally, a chi-square test was utilized to compare the outcomes after delivery between the two groups. The significance level for establishing relationships was set at less than 0.05. Data analysis was performed using SPSS version 24 software.
| Variable | Foley's catheter and oral misoprostol | Oral misoprostol alone | P-Value | |
| Age (year) | 27.11 ± 3.88 | 26.46 ± 4.95 | 0.26 | |
| Gestational age at delivery (week) | 40.03 ± 4.12 | 39.63 ± 3.77 | 0.44 | |
| BMI (kg/m 2 ) | < 25 | 65 (50) | 62 (49.6) | 0.88 |
| 25 - 30 | 44 (33.85) | 40 (32.0) | ||
| > 30 | 21(16.15) | 23 (18.4) | ||
| Labor induction cause | Post term | 31 (23.85) | 30 (24.0) | 0.99 |
| High BP | 22 (16.92) | 23 (18.4) | ||
| LP | 22 (16.92) | 21 (16.8) | ||
| Preeclampsia | 17 (13.08) | 15 (12.0) | ||
| GDM | 15 (11.54) | 14 (11.2) | ||
| Vaginal bleeding | 11 (8.46) | 11 (8.8) | ||
| Decreased fetal movement | 12 (9.23) | 11 (8.8) | ||
| Variable | Foley's catheter and oral misoprostol | Oral misoprostol alone | P.Value |
| Time from the intervention to delivery (h) | 18.01 ± 7.66 | 26 ±6.93 | < 0.001 |
| Bishop score prior induction | 1.76 ± 0.91 | 1.54 ± 1.15 | 0.1 |
| Bishop score in 6 hours | 2.32 ± 1.63 | 1.63 ± 0.51 | < 0.001 |
| Bishop score in 12 hours | 5.72 ± 2.37 | 2.94 ± 1.29 | 0.02 |
| Required time to start the active phase (h) | 12.62 ± 9.40 | 18.69 ± 8.53 | < 0.001 |
| Time needed to initiate labor time (h) | 16.91 ± 9.95 | 24.30 ± 5.33 | < 0.001 |
| Oxytocin dose | 10.37 ± 4.83 | 11.61 ± 3.94 | 0.001 |
| Variable | Foley's catheter and oral misoprostol | Oral misoprostol alone | P.Value | |
| Delivery type | Natural | 110 (84.62) | 91 (79.13) | 0.26 |
| Cesarean | 20 (15.38) | 24 (20.87) | ||
| Delivery time | <24 hours | 115 (88.46) | 32 (25.6) | <0.001 |
| > 24 hours | 15 (11.54) | 93 (74.4) | ||
| Uterine Tachysystole | Yes | 5 (3.85) | 22 (17.6) | <0.001 |
| No | 125 (96.15) | 103 (82.4) | ||
| Bleeding after delivery | Yes | 15 (11.54) | 11 (8.80) | 0.47 |
| No | 115 (88.46) | 114 (91.20) | ||
| NICU hospitalization | Yes | 2 (1.54) | 10 (8.0) | 0.015 |
| No | 128 (98.46) | 115 (92.0) | ||
| Apgar minute 1 | Abnormal | 2 (1.54) | 5 (4.0) | 0.23 |
| Normal | 128 (98.46) | 120 (96.0) | ||
| Apgar minute 5 | Abnormal | 0 | 6 (4.8) | 0.01 |
| Normal | 130 (100) | 119 (95.2) | ||
| Meconium presence | Yes | 2 (1.54) | 9 (7.2) | 0.03 |
| No | 128 (98.46) | 116 (92.8) | ||
Results
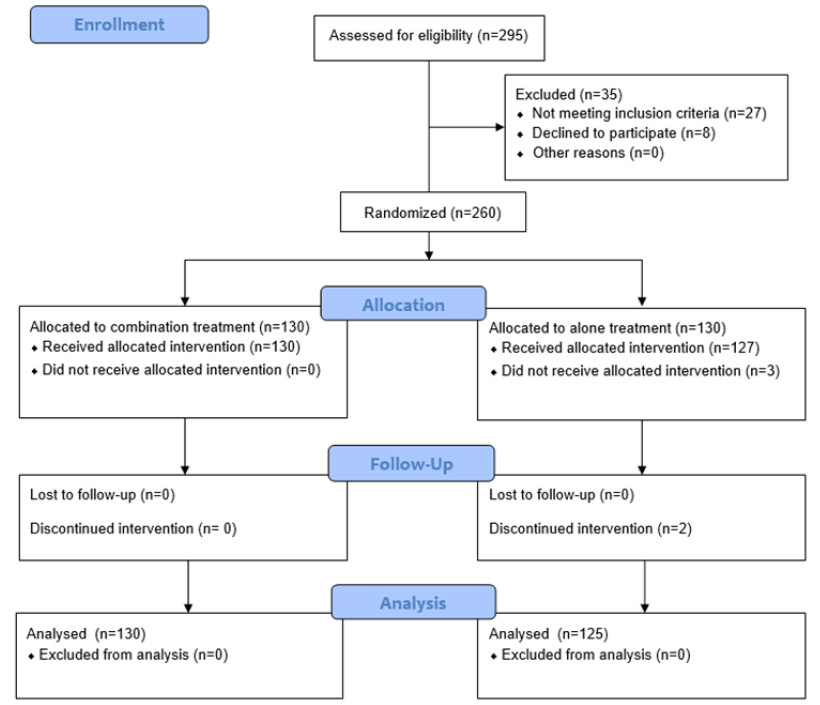
A total of 295 participants were initially assessed for eligibility. Among them, 27 patients did not meet the inclusion criteria, and an additional 8 patients declined to participate. Subsequently, 260 participants were randomly allocated to either the combination treatment or misoprostol alone, with 130 patients assigned to each group. Notably, 3 patients in the misoprostol alone group did not receive their allocated treatment, and 2 patients discontinued the intervention. The discontinuation of the intervention in two cases was due to the preferences expressed by the patients or their partners. In the final analysis, 130 patients from the combination treatment group and 125 patients from the misoprostol alone treatment group were included.
In Table 1, we conducted a comparison of the baseline characteristics of patients within the two investigated treatment groups. As shown, the two groups were homogeneous in regards to age (27.11 ± 3.88 vs. 26.46 ± 4.95, P = 0.26), gestational week (P = 0.44), and BMI (P = 0.88). In both groups, post-term was the main cause of labor induction. However, there was not a significant difference between the two groups in this regard (P = 0.99).
Table 2 compares key variables between the oral misoprostol alone group and the combination of Foley's catheter and oral misoprostol group. The oral misoprostol alone group exhibited significantly higher values in the time from intervention to delivery (P < 0.001), required time to start the active phase (P < 0.001), time needed to initiate labor (P < 0.001), and oxytocin dose (P = 0.001). On the other hand, the combination of Foley's catheter and oral misoprostol group showed significantly higher Bishop scores at 6 hours (P < 0.001) and 12 hours (P = 0.02). There were no significant differences observed between the two groups concerning delivery type (P = 0.26), post-delivery bleeding (P = 0.47), and Apgar score at one minute (P = 0.23). However, a notable discrepancy emerged in the proportion of cases failing to deliver within 24 hours of induction, with the oral misoprostol alone group exhibiting a significantly higher rate compared to the combination treatment group (88.46% vs. 25.6%, P < 0.001). Furthermore, the oral misoprostol alone group demonstrated significantly higher incidences of adverse outcomes, including uterine tachysystole (17.6% vs. 3.85%, P < 0.001), NICU hospitalization (8% vs. 1.54%, P = 0.015), abnormal Apgar score at five minutes (4.8% vs. 0%, P = 0.01), and meconium presence (7.2% vs. 1.54%, P = 0.03), as detailed in Table 3.
Discussion
The current clinical trial was conducted to assess and compare the efficacy and safety of two methods for labor induction, including the combination of misoprostol and Foley catheter methods, versus the use of oral misoprostol alone in women receiving care in the maternity ward. We found that the combination of the Foley catheter and oral misoprostol group showed significantly higher Bishop scores at 6 hours and 12 hours. In contrast, the group receiving oral misoprostol alone demonstrated a significantly higher rate of failing to deliver within 24 hours of induction, and adverse outcomes encompassed uterine tachysystole, NICU hospitalization, abnormal Apgar scores at five minutes, and the presence of meconium.
Regarding the duration from the onset of intervention to birth, our study revealed a noteworthy reduction of approximately 8 hours in the combined group. In contrast to our findings, Lanka and colleagues15 did not observe a significant difference between the two groups. Conversely, Aduloju et al.16 and Nasioudis et al.17 reported consistent findings, indicating a shorter duration in the combined group. This variance could be attributed to differences in study populations, methodologies, or individual patient responses.
In our study, the rate of Bishop score increase was higher in the combined group. This was evident at both 6 and 12 hours post-intervention. Consistent with this, Aduloju et al.16 reported a similar trend, emphasizing the superiority of the combined treatment in advancing cervical ripening. Moreover, the combined group reached the active phase 6 hours earlier. This finding aligns with the increased Bishop score and supports the notion that combined misoprostol treatment accelerates labor progression.
We found that the combined group required a lower oxytocin dose. This aligns with the observed efficiency of the combined treatment, as also noted by Aduloju et al. 16. The reduced need for oxytocin could be associated with the synergistic effects of misoprostol and the Foley catheter, enhancing uterine contractions.
Regarding the frequency of maternal complications, the lower incidence of tachysystole in the combined group suggests potential advantages in terms of safety. However, the comparable risk of hemorrhage warrants careful consideration. A lower incidence of uterine tachysystole in the combination group may be due to the lower doses of misoprostol that are required when combination methods are used17. Contrary to our findings in the two conducted studies in Africa, NICU admission was significantly higher in the combined group16, 18. In the Jain et al. study, the only significant difference between the two groups was found in postpartum hemorrhage and 5-minute Apgar score <7, which was significantly more frequent in the misoprostol alone group19. Comparing our findings with other studies20, 21, discrepancies in outcomes may be attributed to differences in study design, participant demographics, or variations in intervention protocols. The heterogeneity of patient populations and diverse clinical settings may contribute to divergent results.
However, the present study had some limitations. First, the study was conducted solely at Shariati Hospital in Tehran, which may limit the generalizability of the findings to a broader population. Different healthcare settings and patient demographics could influence the outcomes. Secondly, the lack of blinding introduces the potential for bias in outcome assessments and could impact the overall internal validity of the study. Finally, unanticipated dropouts or withdrawals during the study could influence the final sample size and, consequently, the statistical power.
Conclusion
In conclusion, for labor induction, the combined treatment emerges as more appropriate due to its quicker and more impactful results, along with lower complication rates. However, ongoing research and standardization of protocols are imperative to ensure consistent and optimal outcomes across diverse patient populations.
Abbreviations
BMI - Body Mass Index, NICU - Neonatal Intensive Care Unit, PGE1 - Prostaglandin, E1PGE2 - Prostaglandin E2, PROM - Premature Rupture of Membranes, SPSS - Statistical Package for the Social Sciences
Acknowledgments
Deputy of Research and Technology of Tehran University of Medical Sciences approved our study and financially supported the study (Ethic code: IR.TUMS.MEDICINE.REC.1400.764). We would like gratefully acknowledge the medical staff of the Shariati hospital.
Author’s contributions
All authors equally contributed to this work, read and approved the final manuscript.
Funding
Deputy of Research and Technology of Tehran University of Medical Sciences financially supported the study.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Deputy of Research and Technology of Tehran University of Medical Sciences approved the study (Ethic code: IR.TUMS.MEDICINE.REC.1400.764).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Martin
J.A.,
Hamilton
B.E.,
Osterman
M.,
Driscoll
A.,
Drake
P.,
National Center for Health Statistics: Hyattsville (Maryland); 2019.
Google Scholar -
American College of Obstetricians Gynecologists
ACOG practice bulletin no. 107: induction of labor. Obstetrics and Gynecology.
2009;
114
(2)
:
386-97
.
View Article Google Scholar -
Shrem
G.,
Nagawkar
S.S.,
Hallak
M.,
Walfisch
A.,
Isolated oligohydramnios at term as an indication for labor induction: a systematic review and meta-analysis. Fetal Diagnosis and Therapy.
2016;
40
(3)
:
161-73
.
View Article Google Scholar -
Fonseca
M.J.,
Santos
F.,
Afreixo
V.,
Silva
I.S.,
do Céu Almeida
M.,
Does induction of labor at term increase the risk of cesarean section in advanced maternal age? A systematic review and meta-analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology.
2020;
253
:
213-9
.
View Article Google Scholar -
Zenzmaier
C.,
Leitner
H.,
Brezinka
C.,
Oberaigner
W.,
König-Bachmann
M.,
Maternal and neonatal outcomes after induction of labor: a population-based study. Archives of Gynecology and Obstetrics.
2017;
295
(5)
:
1175-83
.
View Article Google Scholar -
Coates
D.,
Homer
C.,
Wilson
A.,
Deady
L.,
Mason
E.,
Foureur
M.,
Induction of labour indications and timing: A systematic analysis of clinical guidelines. Women and Birth ; Journal of the Australian College of Midwives.
2020;
33
(3)
:
219-30
.
View Article Google Scholar -
Alfirevic
Z.,
Keeney
E.,
Dowswell
T.,
Welton
N.J.,
Medley
N.,
Dias
S.,
Which method is best for the induction of labour? A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technology Assessment.
2016;
20
(65)
:
1-583
.
View Article Google Scholar -
Rahimi
M.,
Haghighi
L.,
Baradaran
H.R.,
Azami
M.,
Larijani
S.S.,
Kazemzadeh
P.,
Comparison of the effect of oral and vaginal misoprostol on labor induction: updating a systematic review and meta-analysis of interventional studies. European Journal of Medical Research.
2023;
28
(1)
:
51
.
View Article Google Scholar -
Eikelder
M.L. Ten,
Mast
K.,
Velden
A. Van Der,
Bloemenkamp
K.W.,
Mol
B.W.,
Induction of labor using a Foley catheter or misoprostol: a systematic review and meta-analysis. Obstetrical & Gynecological Survey.
2016;
71
(10)
:
620-30
.
View Article Google Scholar -
Ten Eikelder
M.L.,
Rengerink
K.O.,
Jozwiak
M.,
De Leeuw
J.W.,
De Graaf
I.M.,
Van Pampus
M.G.,
Induction of labour at term with oral misoprostol versus a Foley catheter (PROBAAT-II): a multicentre randomised controlled non-inferiority trial. Lancet.
2016;
387
(10028)
:
1619-28
.
View Article Google Scholar -
Hofmeyr
G.J.,
Oral misoprostol is as safe as Foley catheter for labour induction… or is it?. Lancet.
2016;
387
(10028)
:
1593-4
.
View Article Google Scholar -
McMaster
K.,
Sanchez-Ramos
L.,
Kaunitz
A.M.,
Evaluation of a transcervical Foley catheter as a source of infection: a systematic review and meta-analysis. Obstetrics and Gynecology.
2015;
126
(3)
:
539-51
.
View Article Google Scholar -
Husain
S.,
Husain
S.,
Izhar
R.,
Retracted: Oral misoprostol alone versus oral misoprostol and Foley's catheter for induction of labor: A randomized controlled trial. Journal of Obstetrics and Gynaecology Research.
2017;
43
(8)
:
1270-7
.
View Article Google Scholar -
Visser
L.,
De Graaf
I.M.,
Mol
B.W.,
Combination of foley bulb and vaginal misoprostol compared with vaginal misoprostol alone for cervical ripening and labor induction: a randomized controlled trial. Obstetrics and Gynecology.
2013;
122
(1)
:
156
.
View Article Google Scholar -
Lanka
S.,
Surapaneni
T.,
Nirmalan
P.K.,
Concurrent use of F oley catheter and misoprostol for induction of labor: A randomized clinical trial of efficacy and safety. Journal of Obstetrics and Gynaecology Research.
2014;
40
(6)
:
1527-33
.
View Article Google Scholar -
Aduloju
O.P.,
Akintayo
A.A.,
Adanikin
A.I.,
Ade-Ojo
I.P.,
Combined Foley's catheter with vaginal misoprostol for pre-induction cervical ripening: A randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology.
2016;
56
(6)
:
578-84
.
View Article Google Scholar -
Nasioudis
D.,
Kim
S.W.,
Schoen
C.,
Levine
L.D.,
Maternal and neonatal outcomes with mechanical cervical dilation plus misoprostol compared to misoprostol alone for cervical ripening; a systematic review of literature and metaanalysis. American Journal of Obstetrics & Gynecology MFM.
2019;
1
(2)
:
101-11
.
View Article Google Scholar -
Ugwu
E.,
Onah
H.,
Obi
S.,
Dim
C.,
Okezie
O.,
Chigbu
C.,
Effect of the Foley catheter and synchronous low dose misoprostol administration on cervical ripening: a randomised controlled trial. Journal of Obstetrics & Gynaecology.
2013;
33
(6)
:
572-7
.
View Article Google Scholar -
Jain
S.,
Pasrija
S.,
Kille
H.C.,
Labor induction with combined low-dose oral misoprostol and Foley catheter vs oral misoprostol alone at term gestation - a randomized study. AJOG Global Reports.
2022;
2
(3)
:
100060
.
View Article Google Scholar -
Adhikari
E.H.,
Nelson
D.B.,
McIntire
D.D.,
Leveno
K.J.,
Foley bulb added to an oral misoprostol induction protocol: a cluster randomized trial. Obstetrics and Gynecology.
2020;
136
(5)
:
953-61
.
View Article Google Scholar -
Graham
K.,
Nguyen
M.,
Sit
A.,
Morfin
J.,
Garabedian
M.,
417: Oral misoprostol versus combination of foley bulb catheter and oral misoprostol alone for induction of labor: A randomized controlled trial. American Journal of Obstetrics and Gynecology.
2018;
218
(1)
:
254-5
.
View Article Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 10 (2024)
Page No.: 6852-6858
Published on: 2024-10-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 1641 times
- PDF downloaded - 582 times
- XML downloaded - 45 times
 Biomedpress
Biomedpress


