Abstract
Introduction: Neoadjuvant chemotherapy (NACT) is a prevalent treatment strategy for patients with locally advanced breast cancer (LABC). Achieving a pathologic complete response (pCR) is a critical determinant of favorable outcomes. To enhance response rates, some clinicians have incorporated radiotherapy (RT) prior to surgery.
Methods: This observational cohort study aimed to investigate the initial outcomes and complication rates of preoperative radiotherapy (PRT) in breast cancer (BC) patients who did not achieve a complete response post-NACT. Between January 2017 and January 2020, 216 patients who were clinical T1-3, lymph node-positive, non-metastatic, and received NACT were analyzed. After the final dose of chemotherapy, patients were evaluated clinically and radiologically. Among them, 123 patients were non-complete responders. Of these, 37 patients received PRT according to the guidelines.
Results: Following PRT and surgery, 7 (18.9%) patients showed pCR in the breast and 14 (37.8%) in the axilla. HER2-positive and triple-negative breast cancer (TNBC) patients were significantly associated with complete response following PRT in the breast and axilla (p = 0.029). Post-surgical infection was detected in 11 (29.7%) patients, with factors such as a body mass index greater than 25 kg/m2 significantly affecting surgical site infection rates (p = 0.036). The implant loss rate was 16.7% (n = 2), and there was no grade 3 or higher RT-related skin toxicity.
Conclusion: This study demonstrated that PRT for non-complete responder patients improves pCR rates in the breast and axilla, allowing almost 38% of patients to have successful breast-conserving surgery, and lowering axillary lymph-node dissection rates without an increase in major complications. Clinical trials: ClinicalTrials.gov: NCT05274594.
Introduction
Neoadjuvant chemotherapy (NACT) has significant clinical value in inoperable and locally advanced breast cancer (LABC)1, converting it into an operable tumor2, 3. Not only is NACT now commonly used in patients with largely operable tumors to downstage the primary tumor and increase breast conservation rates (7–12%), but it also moves those patients from having a mastectomy to having breast-conserving surgery (BCS)4. Additionally, NACT shows the impact of systemic therapies on the biology of breast tumors5, such as additional Capecitabine adjuvant therapy being effective against human epidermal growth factor receptor 2 (HER2)-negative BC in patients with residual cancer following NACT6. Moreover, previous research has indicated that BC patients who achieved pathological complete response (pCR) after NACT showed significantly better overall survival (OS) and disease-free survival (DFS), particularly for HER2-positive and triple-negative breast cancer (TNBC)7, 8, 9.
In our institution, we have two basic treatment approaches for patients with non-metastatic and lymph node-positive or large breast tumors: either starting with surgery followed by systemic therapy or initiating systemic therapy first as NACT and then followed by surgery. Currently, NACT is widely used specifically for selected groups of BC patients, such as those with HER2-positive or TNBC subtypes, even for early-stage (stage II and stage I-lymph node positive) patients. NACT aims to achieve a complete response in the breast or in axillary lymph nodes in order to preserve the breast and/or axilla. As the goal is achieving complete response, several physicians have started considering administering radiotherapy (RT) before surgery to increase the complete response rate, which is called preoperative radiotherapy (PRT). Historically, PRT dates back to the late 1940s for BC10, but it has been a hot topic, especially in the last years.
Recently, studies have been conducted on NACT patients irrespective of their response during or at the end of chemotherapy treatment. To our knowledge, this is the first clinical study focusing specifically on NACT patients who did not achieve a clinical complete response (cCR) at the end of their chemotherapy treatment. In this single-center observational cohort study, we aim to present the initial results and complication rates of PRT administration in non-complete responder patients after NACT.
Methods
Study Design
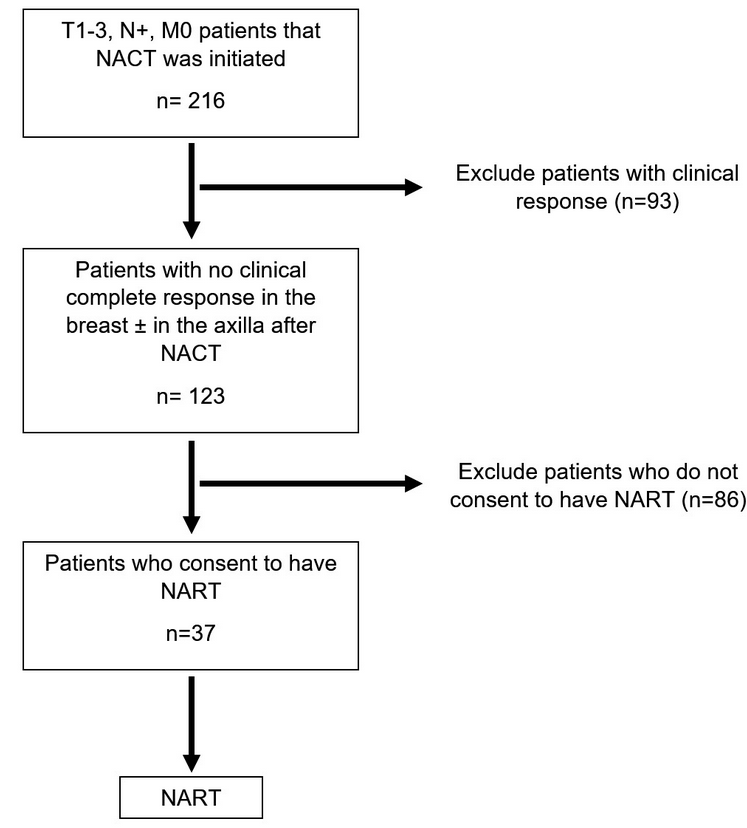
This retrospective observational cohort study was conducted between January 2017 and January 2020 and was registered in the database of clinicaltrials.gov (NCT05274594). The study was approved by the local ethics committee of Istanbul University, Istanbul Faculty of Medicine (No: 2021-536891). Initially, 216 BC patients who were T1-3, N+, M0, and to whom NACT was initiated were followed up. Later, 93 patients were excluded due to their clinical response. The study continued with 123 patients who had no cCR in the breast and/or axilla after NACT. Eighty-six patients who didn’t consent to have NART were excluded. Finally, 37 patients who received NACT were enrolled in the study depending on their order of admission. Every participant signed a written informed consent prior to enrolling in the study.
NACT Treatment Protocol
The NACT regimen was 4 cycles of anthracycline and cyclophosphamide with 12 cycles of a taxane-based regimen. Trastuzumab for 12 months was also added if pathologically HER2-positive. This protocol was the same as planned for the postoperative protocol and it was decided by the medical oncologist of the patient. Within the molecular subtype grouping, luminal A patients were defined as hormone receptor-positive, HER2-negative with low Ki-67 (< 20%) ratios, and luminal B patients were defined as hormone receptor-positive, HER2-negative with high Ki-67 (≥ 20%) ratios. All HER2-positive patients were grouped as HER2-positive, and all hormone receptor-negative with HER2-negative patients were defined as TNBC.
Severe infection was defined as any surgical site infection that required hospitalization, whereas mild infection was defined as a surgical site infection that was managed as ambulatory treatment. pCR was defined as no residual invasive or in-situ tumor in the final pathology following neoadjuvant treatments. cCR was defined as no sign of residual tumor mass, and the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria were used to determine cCR11. Patients were evaluated for the clinical response after the end of the last PRT (median 6 weeks).
Patient Selection and Evaluation
Initially, clinical T1-3, axillary lymph node-positive (N+) (biopsy-proven), and non-metastatic patients with any molecular subtype were included in the study. After the final dose of their chemotherapy, patients were evaluated clinically by physical examination, mammography, ultrasound, and magnetic resonance imaging (MRI) to evaluate tumor response. Patients who achieved complete response in the breast were excluded from the study. Non-complete responder patients who provided consent for the study received PRT as defined in the PRT protocol section of this study. The flow diagram for patient selection is detailed in Figure 1 . Every patient was discussed in the preoperative multidisciplinary breast surgical oncology meeting before and after neoadjuvant treatment. The method of surgery was discussed in these meetings by a group of expert breast surgeons, plastic and reconstructive surgeons, radiation oncologists, medical oncologists, and radiologists. Patients were individually evaluated for breast conservation, mastectomy, or mastectomy with reconstruction. Likewise, the method of axillary surgery was also discussed in these meetings. The indications for mastectomy were initially multi-centric tumors, large tumor volume relative to breast volume that is not allowing a good cosmetic result, patients with BC-related gene mutations, and extensive in-situ components. Reconstruction with implant was also recommended to all mastectomy patients.
Preoperative Radiotherapy Protocol
Radiotherapy was delivered via 4-6 MV X-ray beam energies and intensity-modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT), or forward planning IMRT (field in field – FinF) treatment techniques. PRT was planned for patients who would have BCS as 42.5 Gy in 16 fractions (with 10 Gy in 5 fractions sequential boost doses), 50 Gy in 25 fractions (with 10 Gy in 5 fractions sequential boost doses), or 50.4 Gy in 28 fractions (with 59.9 Gy in 28 fractions simultaneous integrated boost doses), involving whole breast irradiation plus nodal irradiation (including supraclavicular and axillary lymph nodes - Level I, II, III). If mastectomy was scheduled following PRT, irradiation was planned with the same treatment doses (whole breast irradiation plus nodal irradiation) as in BCS, but boost dose delivery was left to the radiation oncologist’s preference. RT to the mammary internal lymph nodes was delivered at the discretion of the radiation oncologist and according to the tumor characteristics such as clinical stage or tumor location. Surgery was planned six weeks after the end of PRT.
Statistical Analysis
During the statistical analysis, the HER2-positive group and the TNBC group were evaluated together because of the small sample size (total of 11 patients), considering that these two groups are more chemo-sensitive than the hormone-positive groups. Categorical and continuous variables were summarized using descriptive statistics (e.g., median, range, frequency, and percentage) and were compared using the Pearson chi-square or Fisher’s exact tests, respectively. The effects of clinical variables on the pCR, surgical site infection, and implant loss were analyzed by univariate analysis. The statistical level of significance was defined as p < 0.05 with 95% confidence intervals. All statistical analyses were performed using the SPSS program (Version 22.0, SPSS Inc., Chicago, IL, USA).
| Total (n = 37) | ||
| n | % | |
| Median Age | 50 (28 - 63) | |
| Age Group | ||
| ≤ 50 | 21 | 56.8 |
| > 50 | 16 | 43.2 |
| Body Mass Index (kg/m 2 ) | ||
| Normal (18.5 - 24.9) | 11 | 29.7 |
| Overweight (25 - 29.9) | 12 | 32.4 |
| Obese (≥ 30) | 14 | 37.8 |
| AJCC Clinical T Stage | ||
| II | 22 | 59.5 |
| III | 15 | 40.5 |
| AJCC Clinical N Stage | ||
| I | 31 | 83.8 |
| II | 6 | 16.2 |
| AJCC Clinical Stage | ||
| II-B | 18 | 48.6 |
| III-A | 19 | 51.4 |
| ER | ||
| Positive | 30 | 81.1 |
| Negative | 7 | 18.9 |
| PR | ||
| Positive | 28 | 75.7 |
| Negative | 9 | 24.3 |
| HER2 | ||
| Positive | 9 | 24.3 |
| Negative | 28 | 75.7 |
| Molecular Subtype | ||
| Luminal-A+B * | 26 | 70.3 |
| HER2-positive/TNBC § | 11 | 29.7 |
| NART dose | ||
| 50 Gy in 25 fr (10 Gy in 5 fr boost in 1 patient) | 18 | 48.6 |
| 42.5 Gy in 16 fr (10 Gy in 5 fr boost in 3 patient) | 12 | 32.4 |
| 50.4 Gy in 28 fr with boost (59.9 Gy in 28 fr SIB) | 7 | 19 |
| Median NART Time | 6 (4 - 8) weeks | |
| Surgery (Breast) | ||
| Mastectomy + Implant Reconstruction | 12 | 32.4 |
| Mastectomy | 11 | 29.7 |
| BCS | 14 | 37.8 |
| Surgery (Axilla) | ||
| SLNB | 21 | 56.8 |
| ALND | 16 | 43.2 |
| Surgery (Breast + Axilla) | ||
| BCS + SLNB | 7 | 18.9 |
| BCS + ALND | 7 | 18.9 |
| Mastectomy + SLNB | 14 | 37.8 |
| Mastectomy + ALND | 9 | 24.3 |
| Response to NART (Breast) | ||
| pCR | 7 | 18.9 |
| No pCR | 30 | 81.8 |
| Response to NART (Axilla) | ||
| pCR | 14 | 37.8 |
| No pCR | 23 | 62.2 |
| Response to NART (Breast + Axilla) | ||
| pCR | 5 | 13.5 |
| No pCR | 32 | 86.5 |
| Complication | ||
| Yes | 14 | 37.8 |
| No | 23 | 62.2 |
| Early Radiotherapy Skin Toxicity | ||
| Grade I | 12 | 32.4 |
| Grade II | 7 | 18.9 |
Results
A total of 37 patients were enrolled in the study. The median age of the patients was 50 (28-63), and 21 (56.8%) of them were under 50. Of the 37 patients, 26 (70.3%) had a body mass index (BMI) of ≥ 25 kg/m². Eighteen patients were clinical stage IIA, and 19 were stage IIIA according to The American Joint Committee on Cancer (AJCC) clinical staging, edition 712. All patients received anthracycline, cyclophosphamide, and taxane-based regimens and trastuzumab if HER2-positive. Molecular subtypes for 26 (70.3%) patients were luminal A and luminal B (70.3%) (luminal A n = 7 and luminal B n = 19), and HER2-positive/TNBC for 11 (29.7%) patients (HER2-positive n = 8 and TNBC n = 3). Surgical treatment was BCS for 14 (37.8%) patients, mastectomy for 11 (29.7%) patients, and mastectomy with implant reconstruction for 12 (32.4%) patients of which two were prepectoral and 10 were subpectoral implants. Twenty-one (56.8%) patients underwent sentinel lymph node biopsy only and 16 (43.2%) had axillary lymph node dissection (ALND). Patient demographics, clinical, and pathological features are presented in Table 1.
Following NACT, PRT, and surgery, pCR was achieved for 7 (18.9%) patients in the breast and for 14 (37.8%) patients in the axilla (Table 2). Of the 37 patients in our cohort that were still clinically ineligible for BCS at the end of NACT, the overall conversion rate to BCS was 37.8% (n = 14) after PRT. Nine patients were luminal subtypes and HER2-negative (conversion rate 34.6%), and 5 were HER2-positive/TNBC type (conversion rate 34.6% vs. 45.5% for luminal and HER2-positive/TNBC type, respectively; p = 0.4). Additionally, for the study group that had a partial clinical response following NACT, only progesterone receptor negativity (p = 0.008) and HER2-positive/TN patients were significantly relevant with complete response in the breast and axilla (p=0.029) following PRT. PRT doses were given in 18 patients as 50 Gy in 25 fractions (one patient received a boost dose of 10 Gy in 5 fractions), 42.5 Gy in 16 fractions (3 patients received a boost dose of 10 Gy in 5 fractions) in 12 patients, and 50.4 Gy in 28 fractions (with a tumor simultaneous integrated boost of 59.9 Gy in 28 fractions) in 7 patients.
| Pathologic Response (Breast) | |||||||
| pCR (n = 7; %18.9) | No-pCR (n = 30; %81.1) | Total (n = 37) | p-value | ||||
| n | % | n | % | n | % | ||
| Molecular Subtype | 0.086 | ||||||
| Luminal A | 2 | 29 | 5 | 71 | 7 | 18.9 | |
| Luminal B * | 1 | 5.3 | 18 | 94.7 | 19 | 51.4 | |
| HER2-positive/TNBC § | 4 | 36.4 | 7 | 63.6 | 11 | 29.7 | |
| Pathologic Response (Axilla) | |||||||
| pCR (n = 14; %37.8) | No-pCR (n = 23; %62.2) | Total (n = 37) | p-value | ||||
| n | % | n | % | n | % | ||
| Molecular Subtype | 0.025 ɤ | ||||||
| Luminal A | 0 | 0 | 7 | 30.4 | 7 | 18.9 | |
| Luminal B * | 7 | 36.8 | 12 | 63.2 | 19 | 51.4 | |
| HER2- positive/TNBC § | 7 | 63.6 | 4 | 36.4 | 11 | 29.7 | |
| Pathologic Response (Breast + Axilla) | |||||||
| pCR (n = 5; %13.5) | No-pCR (n = 32; %86.5) | Total (n = 37) | p-value | ||||
| n | % | n | % | n | % | ||
| Molecular Subtype | 0.029 ɤ | ||||||
| Luminal A | 0 | 0 | 7 | 100 | 7 | 18.9 | |
| Luminal B * | 1 | 5.3 | 18 | 94.7 | 19 | 51.4 | |
| HER2- positive/TNBC § | 4 | 36.4 | 7 | 63.6 | 11 | 29.7 | |
Post-surgical infection was detected in 11 (29.7%) patients (Table 3). Eight of them were severe and three were mild. Factors significantly affecting surgical site infection were a BMI greater than 25 kg/m² (p=0.036) and having ALND (p = 0.030). Four out of 12 patients that underwent mastectomy with implant reconstruction had an infection (3 of them were severe), and 2 of them were associated with ischemia. The implant loss rate was 16.7% (n = 2). Delivery schedules of PRT (hypofractionation or conventional fractionation) did not affect complication rates. Grade 1 and 2 RT-related skin toxicity rates are presented in Table 1 , while there was no grade 3 or higher skin toxicity. During the median 36 months of follow-up, there was only one (2.7%) local recurrence, which occurred in a mastectomy patient at the 12th month of follow-up. This patient had skin nodules and did not respond to NACT prior to PRT. Distant metastasis was detected in four patients (10.8%). There was no mortality.
| Infection | |||||||
| Yes (n = 11) | No (n = 26) | Total (n = 37) | |||||
| n | % | n | % | n | % | p-value | |
| Median Age | 51 (28 - 63) | 46 (30 - 63) | 50 (28 - 63) | 0.665 | |||
| Age Group | 0.367 | ||||||
| ≤ 50 | 5 | 23.8 | 16 | 76.2 | 21 | 56.8 | |
| > 50 | 6 | 37.5 | 10 | 62.5 | 16 | 43.2 | |
| Body Mass Index (kg/m 2 ) | 0.036* | ||||||
| Normal (18.5 - 24.9) | 0 | 0.0 | 11 | 100.0 | 11 | 29.7 | |
| Overweight (25 - 29.9) | 5 | 41.7 | 7 | 58.3 | 12 | 32.4 | |
| Obese (≥ 30) | 6 | 42.9 | 8 | 57.1 | 14 | 37.8 | |
| AJCC Clinical T Stage | 0.736 | ||||||
| II | 7 | 31.8 | 15 | 68.2 | 22 | 59.5 | |
| III | 4 | 26.7 | 11 | 73.3 | 15 | 40.5 | |
| AJCC Clinical N Stage | 1.000 | ||||||
| I | 9 | 29.0 | 22 | 71.0 | 31 | 83.8 | |
| II | 2 | 33.3 | 4 | 66.7 | 6 | 16.2 | |
| AJCC Clinical Stage | 1.000 | ||||||
| II-B | 5 | 27.8 | 13 | 72.2 | 18 | 48.6 | |
| III-A | 6 | 31.6 | 13 | 68.4 | 19 | 51.4 | |
| NART time | 1.000 | ||||||
| < 5 weeks | 3 | 25.0 | 9 | 75.0 | 12 | 32.4 | |
| ≥ 5 weeks | 8 | 32.0 | 17 | 68.0 | 25 | 67.6 | |
| Response to NART (Breast + Axilla) | 0.623 | ||||||
| pCR | 2 | 40 | 3 | 60 | 5 | 13.5 | |
| No pCR | 9 | 28.1 | 23 | 71.9 | 32 | 86.5 | |
| Surgery (Breast + Axilla) | 0.119 | ||||||
| BCS + SLNB | 1 | 14.3 | 6 | 85.7 | 7 | 18.9 | |
| BCS + ALND | 3 | 42.9 | 4 | 57.1 | 7 | 18.9 | |
| Mastectomy + SLNB | 2 | 14.3 | 12 | 85.7 | 14 | 37.8 | |
| Mastectomy + ALND | 5 | 55.6 | 4 | 44.4 | 9 | 24.3 | |
Discussion
NACT is routinely used in breast centers for LABC. However, years before the introduction of NACT, the sole treatment method for this group of patients was PRT. One of the earliest uses of this treatment modality was in the late 1940s. The aim of this treatment was the same as NACT: to downstage the tumor in order to achieve better surgical outcomes. Recently, PRT can be used concomitantly with NACT13, specifically for hormone receptor-positive tumors that are less likely to achieve a complete response, or after chemotherapy for non-responder patients. In this study, we administered PRT for patients who didn’t show a cCR following NACT. As the IMRT technique demonstrates more homogeneous and targeted dose distribution with lower doses and reduces acute toxicities14, we prefer to use this protocol as PRT.
With the initiation of new drugs, new combination regimens, and targeted therapies, we achieve pCR as high as 70%15. However, there are still many patients lacking pCR after neoadjuvant treatment, especially with the luminal molecular subtypes and some patients with HER2-positive and TNBC subtypes. At this point, PRT is an option. Mladenovic, J et al. reported a pCR rate of 15% in their series of 134 patients diagnosed with LABC (stage IIIA and IIIB) and received PRT followed by mastectomy16. In another study by Calitchi et al., pCR in the breast has been reported as 11%17. A phase III randomized trial compared concomitant NACT + PRT with PRT alone in 271 LABC (stage IIB and IIIA) patients18. In this study, Semiglazov et al. reported breast pCR rates as 29.1% for the NACT + PRT group and 19.4% for the PRT-only group (p < 0.05)18. Riet F.G. et al. published their 32 years of follow-up results for LABC patients (stage IIA-IIIC) treated by preoperative whole breast, ipsilateral axillary, supraclavicular, and internal mammary chain PRT followed by modified radical mastectomy19. The pCR rate in both the tumor and lymph nodes was 10%, and it was 26% for the TNBC subtype. Finally, Nichols et al. conducted a phase II prospective trial for early-stage BC patients (T1-2, N0) who underwent preoperative accelerated partial breast irradiation (APBI) followed by BCS in 27 patients20. A 15% pCR was detected in the breast. As stated in these studies, a pCR rate between 10% and 20% is achieved only by PRT, depending on the RT technique and patient selection. These previously published series did not evaluate clinical responses solely to NACT as complete or partial response, so the benefit of additional PRT is missing. The pCR could either be achieved as a result of NACT or PRT. In our series, we presented a pCR rate of 18.9% in the breast and 37.8% in the axilla. At this point, we should highlight that these pCR rates are for patients that clinically did not achieve a complete response after NACT. This means that the addition of PRT for these patients demonstrates an additional advantage for surgery, sparing more patients from mastectomy and from ALND. The pCR rate was significantly higher for HER2-positive and TNBC subtypes when compared with luminal subtypes (36.4% vs 11.5% in the breast; p = 0.02 and 63.6% vs 27% in the axilla; p=0.025 for HER2-positive/TNBC subtype and luminal subtype, respectively). Even though NACT could affect the pCR rates of these patients, we carefully selected the patients by discussing it in a multidisciplinary meeting with the help of ultrasound, mammography, MRI, and PET-CT if needed.
The timing of the surgery after PRT is an important factor in achieving pCR. The general attitude of physicians is to delay surgery for 3 weeks after hypofractionated RT and 6 weeks after standard RT. For the study group, the median time between the end of PRT and surgery was 6 weeks. Depending on the fractionation, it ranged from 4 weeks to 8 weeks for our cohort. Delaying surgery longer than this deserves investigation, aiming to increase pCR and decrease RT-related complications.
A prominent advantage of NACT is the conversion of patients from mastectomy to BCS. This is also one of the primary aims of PRT. Bollet et al. investigated the effect of RT combined with hormonotherapy initiated before surgery for postmenopausal hormone receptor-positive patients who were ineligible for BCS at first admission21. They reported a 63% rate of conversion to BCS at a median of 8 weeks’ time interval between PRT and surgery. In another phase II trial by the same author that presents the results of concurrent PRT and NACT for early-stage BC patients13, the conversion rate was reported as 69%. Of the 37 patients in our cohort who were still clinically ineligible for BCS at the end of NACT, the overall conversion rate to BCS was 37.8% (n = 14) after PRT. Nine patients were non-HER2 luminal subtypes (conversion rate 34.6%) and five were HER2-positive/TNBC types (conversion rate 34.6% vs 45.5% for luminal and HER2-positive/TNBC subtypes, respectively; p = 0.4).
As is known, RT may affect surgical outcomes and cause complications due to radiation-induced injury, specifically during the early post-surgical period. Postoperative complications occurred in 14 patients (38%). Eight (22%) were severe surgical site infections, three (8%) were seroma only, and three (8%) were mild surgical site infections. Of the 12 patients who underwent mastectomy and breast reconstruction with implants, there have been only two (16.7%) implant losses due to skin ischemia caused by severe surgical site infections, and both patients had sub-pectoral implants. The first patient presented with skin ischemia in the first postoperative month and received antibiotics for 40 days but lost her implant 75 days after the operation. The second patient experienced it in the third postoperative week. This patient was also managed conservatively with antibiotics for a month, but the implant was removed due to resistant infection that affected the patient’s clinic. Additionally, one of the patients with implant loss was a smoker. The only significant factor affecting surgical site infection was BMI≥25 kg/m2, which applied to all patients with infection. As presented here, implant loss was not directly correlated with PRT and could be multifactorial. Besides, this rate is comparable with the published literature for patients who only received NACT as initial treatment before surgery22. Ruvalcaba-Limon et al. published a case-control analysis about surgical complications after preoperative concomitant chemo-radiation23. Of the 360 patients, 165 (45.8%) developed a wound complication (infection ± flap necrosis), of which 60 (16.6%) had surgical site infections and 80 (22.2%) had seroma. They also reported a 10% rate of surgical site infection for patients who had not received any treatment before surgery. Skinner et al.24 and Formenti et al.25 reported postsurgical complication rates of 41% and 14%, respectively, with chemoradiation. In the PAPBI trial26 and the Cambridge IMRT trial27 that investigate the outcomes of PRT followed by BCS, authors reported an 11% postoperative infection rate in the PAPBI trial and 19.7% in the latter trial. A phase II trial reporting surgical site complications for patients that only administered PRT was published by Nichols et al.20. They presented a 15% complication rate that was all grade 3 seromas. It is advised to delay surgery for 4 to 6 weeks to minimize the unfavorable effects of RT on complications. We only observed early grade I and II skin reactions after PRT in 19 patients. The median time delay in our series was 6 weeks. A future study to postpone surgery more than 6 weeks with the initiation of endocrine therapy for only hormone receptor-positive patients is planned to determine if this approach further lowers complication rates and increases pCR rates.
There are several limitations to this study. First, the study is a prospective, single-center study with a relatively small sample size of 37 patients. While we believe that the data from this cohort provide important insights into preoperative radiotherapy (PRT) in non-responder breast cancer patients post-NACT, the small sample size reduces the statistical power and limits the generalizability of the findings. A larger and more diverse patient population would provide greater variability and strengthen the applicability of the results across broader demographic and clinical settings. Additionally, the reliance on imaging and physical examination, rather than tumor bed biopsy, to evaluate patients may introduce some variability in assessing residual disease. However, this approach is consistent with daily clinical practice, as patients are routinely evaluated using ultrasound, mammography, MRI, and PET-CT, following RECIST 1.1 criteria. This makes the methodology highly practical and applicable in real-world settings, even though biopsy-based evaluation might offer additional pathological confirmation.
Future research should address these limitations by employing a multicenter prospective design with a larger, more diverse patient cohort. Such studies could validate our findings, enhance their generalizability, and further elucidate the role of PRT in this patient population. Additionally, incorporating tumor bed biopsies as part of the assessment protocol could provide a more robust evaluation of treatment responses.
Conclusions
To conclude, this is the first clinical trial to specifically evaluate the sole effect of PRT (preoperative radiotherapy) on patient outcomes, such as pCR (pathologic complete response) rates, and BCS (breast-conserving surgery) conversion rates in LABC (locally advanced breast cancer) patients who initially received NACT (neoadjuvant chemotherapy) and did not achieve cCR (clinical complete response) at the end of their primary chemotherapy. This study demonstrated that administering RT (radiotherapy) prior to surgery for non-complete responder patients to NACT improves pCR rates in both the breast and axilla, allowing almost 38% of the patients to be converted to BCS and reducing ALND (axillary lymph node dissection) rates without increasing major complications. These findings highlight the potential impact of PRT on optimizing surgical outcomes and contribute valuable knowledge to the existing literature on treatment strategies for non-complete responders. While this study focuses on short-term outcomes, the long-term clinical and survival implications of RT, including the potential abscopal effect, will be explored in future analyses with extended follow-up.
ENDPOINTS
Abbreviations
BC: Breast Cancer, BCS: Breast-Conserving Surgery, BMI: Body Mass Index, cCR: Clinical Complete Response, DFS: Disease-Free Survival, HER2: Human Epidermal Growth Factor Receptor 2, IMRT: Intensity-Modulated Radiotherapy, LABC: Locally Advanced Breast Cancer, NACT: Neoadjuvant Chemotherapy, OS: Overall Survival, PAPBI: Preoperative Accelerated Partial Breast Irradiation, PET-CT: Positron Emission Tomography - Computed Tomography, pCR: Pathologic Complete Response, PRT: Preoperative Radiotherapy, RECIST: Response Evaluation Criteria in Solid Tumors, RT: Radiotherapy, SPSS: Statistical Package for the Social Sciences, TNBC: Triple-Negative Breast Cancer, VMAT: Volumetric Modulated Arc Therapy
Acknowledgments
Statistical analysis: We would like to express our appreciation to MSc. Atilla Bozdogan for his meticulous statistical analysis for this study.
Author’s contributions
MM: study concepts and design, data acquisition, quality control of data and algorithms, data analysis and interpretation, statistical analysis and manuscript preparation, editing and review. SE, EO, KI, MT, NC, and NZ: study design, data acquisition, quality control of data and algorithms, data analysis and interpretation, editing and review. AA, AI, VO, data acquisition, quality control of data and algorithms, manuscript editing and review. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Local ethical committee of the home institution approved the study and informed consent was obtained from all individual participants included in the study. This retrospective observational cohort study was conducted between January 2017 and January 2020 and approved by the local ethical committee of the home institution (Approval No: 2021-536891).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Schegerin
M.,
Tosteson
A.N.,
Kaufman
P.A.,
Paulsen
K.D.,
Pogue
B.W.,
Prognostic imaging in neoadjuvant chemotherapy of locally-advanced breast cancer should be cost-effective. Breast Cancer Research and Treatment.
2009;
114
(3)
:
537-47
.
View Article PubMed Google Scholar -
Charfare
H.,
Limongelli
S.,
Purushotham
A.D.,
Neoadjuvant chemotherapy in breast cancer. British Journal of Surgery.
2005;
92
(1)
:
14-23
.
View Article PubMed Google Scholar -
Costa
S.D.,
Loibl
S.,
Kaufmann
M.,
Zahm
D.M.,
Hilfrich
J.,
Huober
J.,
Neoadjuvant chemotherapy shows similar response in patients with inflammatory or locally advanced breast cancer when compared with operable breast cancer: a secondary analysis of the GeparTrio trial data. Journal of Clinical Oncology.
2010;
28
(1)
:
83-91
.
View Article PubMed Google Scholar -
Schott
A.F.,
Hayes
D.F.,
Defining the benefits of neoadjuvant chemotherapy for breast cancer. Journal of Clinical Oncology.
2012;
30
(15)
:
1747-9
.
View Article PubMed Google Scholar -
Vaidya
J.S.,
Massarut
S.,
Vaidya
H.J.,
Alexander
E.C.,
Richards
T.,
Caris
J.A.,
Rethinking neoadjuvant chemotherapy for breast cancer. BMJ (Clinical Research Ed.).
2018;
360
:
j5913
.
View Article PubMed Google Scholar -
Masuda
N.,
Lee
S.J.,
Ohtani
S.,
Im
Y.H.,
Lee
E.S.,
Yokota
I.,
Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. The New England Journal of Medicine.
2017;
376
(22)
:
2147-59
.
View Article PubMed Google Scholar -
von Minckwitz
G.,
Blohmer
J.U.,
Costa
S.D.,
Denkert
C.,
Eidtmann
H.,
Eiermann
W.,
Response-guided neoadjuvant chemotherapy for breast cancer. Journal of Clinical Oncology.
2013;
31
(29)
:
3623-30
.
View Article PubMed Google Scholar -
Spring
L.M.,
Fell
G.,
Arfe
A.,
Sharma
C.,
Greenup
R.,
Reynolds
K.L.,
Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clinical Cancer Research.
2020;
26
(12)
:
2838-48
.
View Article PubMed Google Scholar -
Krishnan
Y.,
Alawadhi
S.A.,
P S
S.,
Gopal
M.,
Thuruthel
S.,
Pathological responses and long-term outcome analysis after neoadjuvant chemotheraphy in breast cancer patients from Kuwait over a period of 15 years. Annals of Saudi Medicine.
2013;
33
(5)
:
443-50
.
View Article PubMed Google Scholar -
Pfahler
G.E.,
Keefer
G.P.,
The pub, the value, and the technique of preoperative and postoperative X-ray treatment in carcinoma of the breast. Surgery, Gynecology {&}amp; Obstetrics.
1947;
85
(1)
:
35-46
.
PubMed Google Scholar -
Eisenhauer
E.A.,
Therasse
P.,
Bogaerts
J.,
Schwartz
L.H.,
Sargent
D.,
Ford
R.,
New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer (Oxford, England).
2009;
45
(2)
:
228-47
.
View Article PubMed Google Scholar -
Edge
S.B.,
Compton
C.C.,
The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology.
2010;
17
(6)
:
1471-4
.
View Article Google Scholar -
Bollet
M.A.,
Belin
L.,
Reyal
F.,
Campana
F.,
Dendale
R.,
Kirova
Y.M.,
Preoperative radio-chemotherapy in early breast cancer patients: long-term results of a phase II trial. Radiotherapy and Oncology : Journal of the European Society for Therapeutic Radiology and Oncology.
2012;
102
(1)
:
82-8
.
View Article PubMed Google Scholar -
Lee
H.H.,
Hou
M.F.,
Chuang
H.Y.,
Huang
M.Y.,
Tsuei
L.P.,
Chen
F.M.,
Intensity modulated radiotherapy with simultaneous integrated boost vs. conventional radiotherapy with sequential boost for breast cancer - A preliminary result. The Breast.
2015;
24
(5)
:
656-60
.
View Article PubMed Google Scholar -
Özkurt
E.,
Sakai
T.,
Wong
S.M.,
Tukenmez
M.,
Golshan
M.,
Survival Outcomes for Patients With Clinical Complete Response After Neoadjuvant Chemotherapy: Is Omitting Surgery an Option?. Annals of Surgical Oncology.
2019;
26
(10)
:
3260-8
.
View Article PubMed Google Scholar -
Mladenovic
J.,
Susnjar
S.,
Tanic
M.,
Jankovic
R.,
Karadzic
K.,
Gavrilovic
D.,
Tumor response and patient outcome after preoperative radiotherapy in locally advanced non-inflammatory breast cancer patients. Journal of the Balkan Union of Oncology.
2017;
22
(2)
:
325-33
.
PubMed Google Scholar -
Calitchi
E.,
Kirova
Y.M.,
Otmezguine
Y.,
Feuilhade
F.,
Piedbois
Y.,
Le Bourgeois
J.P.,
Long-term results of neoadjuvant radiation therapy for breast cancer. International Journal of Cancer.
2001;
96
(4)
:
253-9
.
View Article PubMed Google Scholar -
Semiglazov
V.F.,
Topuzov
E.E.,
Bavli
J.L.,
Moiseyenko
V.M.,
Ivanova
O.A.,
Seleznev
I.K.,
Primary (neoadjuvant) chemotherapy and radiotherapy compared with primary radiotherapy alone in stage IIb-IIIa breast cancer. Annals of Oncology : Official Journal of the European Society for Medical Oncology.
1994;
5
(7)
:
591-5
.
View Article PubMed Google Scholar -
Riet
F.G.,
Fayard
F.,
Arriagada
R.,
Santos
M.A.,
Bourgier
C.,
Ferchiou
M.,
Preoperative radiotherapy in breast cancer patients: 32 years of follow-up. European Journal of Cancer (Oxford, England).
2017;
76
:
45-51
.
View Article PubMed Google Scholar -
Nichols
E.,
Kesmodel
S.B.,
Bellavance
E.,
Drogula
C.,
Tkaczuk
K.,
Cohen
R.J.,
Preoperative Accelerated Partial Breast Irradiation for Early-Stage Breast Cancer: Preliminary Results of a Prospective, Phase 2 Trial. International Journal of Radiation Oncology, Biology, Physics.
2017;
97
(4)
:
747-53
.
View Article PubMed Google Scholar -
Bollet
M.A.,
Kirova
Y.M.,
Antoni
G.,
Pierga
J.Y.,
Sigal-Zafrani
B.,
Laki
F.,
Responses to concurrent radiotherapy and hormone-therapy and outcome for large breast cancers in post-menopausal women. Radiotherapy and Oncology : Journal of the European Society for Therapeutic Radiology and Oncology.
2007;
85
(3)
:
336-45
.
View Article PubMed Google Scholar -
Vieira
R.A.,
Ribeiro
L.M.,
Carrara
G.F.,
Abrahão-Machado
L.F.,
Kerr
L.M.,
Nazário
A.C.,
Effectiveness and Safety of Implant-Based Breast Reconstruction in Locally Advanced Breast Carcinoma: A Matched Case-Control Study. Breast Care (Basel, Switzerland).
2019;
14
(4)
:
200-10
.
View Article PubMed Google Scholar -
Ruvalcaba-Limón
E.,
Robles-Vidal
C.,
Poitevin-Chacón
A.,
Chávez-Macgregor
M.,
Gamboa-Vignolle
C.,
Vilar-Compte
D.,
Complications after breast cancer surgery in patients treated with concomitant preoperative chemoradiation: A case-control analysis. Breast Cancer Research and Treatment.
2006;
95
(2)
:
147-52
.
View Article PubMed Google Scholar -
Skinner
K.A.,
Silberman
H.,
Florentine
B.,
Lomis
T.J.,
Corso
F.,
Spicer
D.,
Preoperative paclitaxel and radiotherapy for locally advanced breast cancer: surgical aspects. Annals of Surgical Oncology.
2000;
7
(2)
:
145-9
.
View Article PubMed Google Scholar -
Formenti
S.C.,
Volm
M.,
Skinner
K.A.,
Spicer
D.,
Cohen
D.,
Perez
E.,
Preoperative twice-weekly paclitaxel with concurrent radiation therapy followed by surgery and postoperative doxorubicin-based chemotherapy in locally advanced breast cancer: a phase I/II trial. Journal of Clinical Oncology.
2003;
21
(5)
:
864-70
.
View Article PubMed Google Scholar -
van der Leij
F.,
Bosma
S.C.,
van de Vijver
M.J.,
Wesseling
J.,
Vreeswijk
S.,
Rivera
S.,
First results of the preoperative accelerated partial breast irradiation (PAPBI) trial. Radiotherapy and Oncology : Journal of the European Society for Therapeutic Radiology and Oncology.
2015;
114
(3)
:
322-7
.
View Article PubMed Google Scholar -
Mukesh
M.B.,
Barnett
G.,
Cumming
J.,
Wilkinson
J.S.,
Moody
A.M.,
Wilson
C.,
Association of breast tumour bed seroma with post-operative complications and late normal tissue toxicity: results from the Cambridge Breast IMRT trial. European Journal of Surgical Oncology.
2012;
38
(10)
:
918-24
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 12 (2024)
Page No.: 6978-6988
Published on: 2024-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
- HTML viewed - 1357 times
- PDF downloaded - 442 times
- XML downloaded - 64 times
 Biomedpress
Biomedpress



