Abstract
Introduction: Breast cancer is a significant global health issue, particularly in women, and is the fifth leading cause of cancer-related deaths in Iran. This study investigates the anticancer effects of naringin and melatonin on SKBR3 (HER2+) and MCF-7 (HER2-) breast cancer cell lines.
Methods: Cell viability and cytotoxicity were assessed using the MTT assay, which measures the reduction of MTT to formazan by mitochondrial dehydrogenase enzymes in viable cells. Cells were treated with various concentrations of naringin and melatonin. Antioxidant enzyme activities of SOD and LDH were measured, and lipid peroxidation was assessed by MDA levels. Statistical analysis was performed using SPSS and GraphPad Prism software, with significance set at p < 0.05.
Results: Both compounds showed a dose-dependent reduction in cell viability and increased cell death in both cell lines. Naringin significantly decreased SOD activity while increasing LDH activity and MDA levels. Similarly, melatonin treatment led to increased cell death, elevated MDA levels, and higher LDH activity, coupled with a decrease in SOD activity.
Conclusion: The findings indicate that naringin and melatonin have potent anticancer properties. Their ability to induce oxidative stress and modulate antioxidant defenses suggests their potential as therapeutic agents in breast cancer treatment. Further research and clinical applications are warranted to explore their efficacy in combating breast cancer. Both compounds exhibit significant anticancer properties against breast cancer cell lines, SKBR3 and MCF-7, making them promising candidates for further study.
Introduction
Breast cancer is a significant global health issue, with substantial physical, psychological, and economic impacts on patients and their families1. It is the most prevalent neoplastic malignancy among women worldwide and stands as the fifth leading cause of cancer-related deaths among women in Iran. Annually, over 2,588 new cases are diagnosed in the country, reflecting the urgent need for more effective treatments2.
Recent advancements in cancer research have highlighted the potential of natural compounds in oncology3. Melatonin, a hormone known primarily for regulating sleep cycles, has garnered attention for its anticancer properties4. This compound not only influences hormonal pathways but also exhibits powerful antioxidant effects, which can inhibit cancer cell proliferation and induce apoptosis5. Similarly, naringin, a flavonoid found in citrus fruits, has shown promise in cancer therapy due to its antioxidant and pro-apoptotic activities6.
Despite the progress in breast cancer treatments, there remains a knowledge gap concerning the precise mechanisms through which melatonin and naringin exert their anticancer effects. This study posits that combining melatonin and naringin can enhance their effectiveness by inducing oxidative stress and apoptosis in breast cancer cells4. Through this research, we aim to provide new insights into their mechanisms of action and explore their potential as novel therapeutic agents.
This study aims to evaluate the anticancer effects of melatonin and naringin on two breast cancer cell lines: SKBR3 (HER2+) and MCF-7 (HER2-). The SKBR3 cell line is frequently used as a control in HER2 assays due to its overexpression of the HER2 receptor, which is associated with aggressive tumor growth. In contrast, the MCF-7 cell line is crucial for studies involving estrogen receptor-positive breast cancer. The investigation focuses on understanding how these natural compounds can inhibit cancer cell growth and promote apoptosis.
Methods
Cell Lines and Culture Conditions
In this study, two breast cancer cell lines, SKBR3 (HER2+) and MCF-7 (HER2-), were utilized to investigate the anticancer and antioxidant effects of naringin and melatonin. These cell lines were procured from the Pasteur Institute of Iran. Cells were cultured in DMEM medium supplemented with 10% fetal bovine serum (FBS) in sterile cell culture flasks. The culture medium was refreshed every 2-3 days, and cells were passaged weekly to maintain optimal growth conditions. Cell passages ranged from 5 to 20 to ensure consistency. Cells were incubated at 37 °C in a humidified atmosphere with 5% CO2 to maintain optimal physiological conditions.
For the treatment experiments, cells were exposed to various concentrations of naringin and melatonin, determined based on the IC50 values, for a duration of 24 hours. This allowed for the assessment of dose-dependent responses. To ensure the accuracy and reproducibility of the results, all experiments were performed in triplicate. Additionally, strict adherence to standard cell culture protocols and quality control measures was maintained throughout the study to minimize any variability and ensure the reliability of the data.
Experimental Groups
The experimental setup included the following groups:
MTT Assay for Cell Viability and IC50 Determination
Cell viability and cytotoxicity were assessed using the MTT assay, a colorimetric assay based on the reduction of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to formazan by mitochondrial dehydrogenase enzymes in viable cells. Cells were treated with logarithmic concentrations of naringin and melatonin (0.1, 1, 10, 100, 500, and 1000 µg/mL) for 24 hours. The absorbance of the formazan product was measured at 571 nm using a spectrophotometer. Cell viability was calculated, and the IC50 value was determined, which represents the concentration required to inhibit cell growth by 50%7.
Assessment of Antioxidant Enzyme Activity
The activities of the antioxidant enzymes superoxide dismutase (SOD) and lactate dehydrogenase (LDH) were measured to evaluate the antioxidant effects of naringin and melatonin on SKBR3 and MCF-7 cells. Cells were treated, detached using trypsin, and centrifuged at 1300 rpm for 7 minutes. The cell pellet was washed twice with cold PBS and lysed with 100 µL of lysis buffer. After centrifugation at 10,000 rpm for 15 minutes at 4 °C, the supernatant was used for enzyme activity assays and protein quantification using the Bradford method.
Superoxide Dismutase (SOD) Activity Assay
SOD activity was measured using a ZELLX SOD assay kit, based on the Beauchamp and Fridovich method with modifications for ELISA reading. The reaction mixture contained 50 µM phosphate buffer (pH 7.2), 0.1 µM EDTA, 13 µM methionine, 75 µM nitro blue tetrazolium (NBT), 0.21 µM riboflavin, and the enzyme supernatant. The absorbance was read at specific wavelengths, comparing samples, blanks, and controls.
Lactate Dehydrogenase (LDH) Activity Assay
LDH activity, indicating cell membrane integrity, was measured using the German Biochemical Society (DGK) standard method and Pars Azmoon kit. The assay measures NADH production during lactate conversion to pyruvate, with a direct relationship to LDH activity, quantified by spectrophotometry.
Lipid Peroxidation Assay
Lipid peroxidation, indicative of membrane damage, was assessed by measuring malondialdehyde (MDA) levels. Cells (3 × 105) were centrifuged at 3000 rpm for 5 minutes. The pellet was resuspended in 1.5 mL of 10% trichloroacetic acid, sonicated, and centrifuged at 10,000 rpm for 10 minutes. The supernatant was mixed with 0.5 mL of 0.5% thiobarbituric acid, incubated at 100 °C for 30 minutes, and the absorbance was measured at 532 nm and 600 nm. MDA concentration was calculated using a molar extinction coefficient (ε = 155 µM⁻¹cm⁻¹).
Statistical Analysis
Data analysis was performed using SPSS (version 19.0, Chicago, IL, USA) and GraphPad Prism 6 software. Results were expressed as mean ± standard deviation (SD) of five independent experiments. Data normality was assessed using the Kolmogorov-Smirnov test. Differences between groups were analyzed using one-way ANOVA followed by Tukey's post-hoc test for pairwise comparisons. Statistical significance was set at (p < 0.05).
Results
Cell Viability and Cytotoxicity Analysis
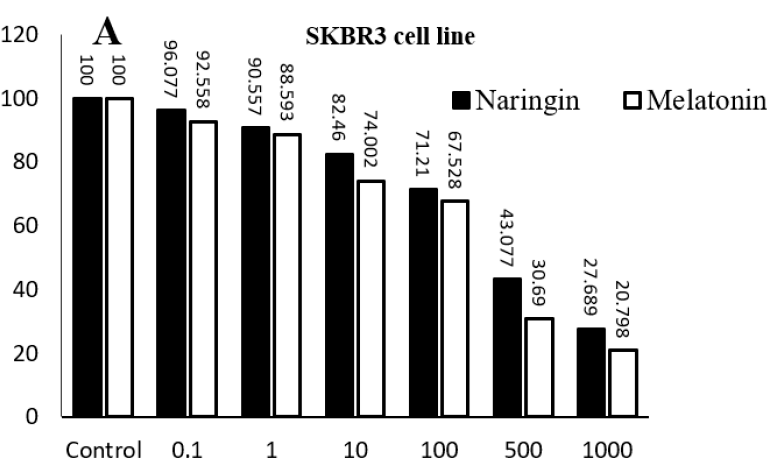
In this study, various concentrations of naringin (0.1 µM, 1 µM, 10 µM, 100 µM, 500 µM, and 1000 µM) and melatonin (0.1 µM, 1 µM, 10 µM, 100 µM, 500 µM, and 1000 µM) were used to assess their cytotoxic effects on MCF-7 (HER2-) and SKBR3 (HER2+) breast cancer cell lines. Following the treatment period, a dose-dependent increase in cell death and cytotoxicity was observed for both compounds across both cell lines. Specifically, in the MCF-7 cell line, increased concentrations of naringin significantly elevated cell death and cytotoxicity compared to the control group. A similar pattern was noted in the SKBR3 cell line, where escalating concentrations of naringin resulted in higher levels of cell death and cytotoxicity. Notably, the impact of varying naringin dosages on cell death was more pronounced in the SKBR3 cell line compared to the MCF-7 cell line. For melatonin, increased concentrations also led to a significant rise in cell death and cytotoxicity in both MCF-7 and SKBR3 cell lines when compared to the control group. The SKBR3 cell line exhibited a more marked response to melatonin treatment, displaying greater cell death and cytotoxicity with increasing concentrations. This trend was similarly observed in the MCF-7 cell line, though to a slightly lesser extent. These findings suggest that both naringin and melatonin possess potent cytotoxic effects on breast cancer cells, with a more pronounced impact on the SKBR3 cell line. The dose-dependent nature of these effects underscores the potential of these compounds as therapeutic agents in breast cancer treatment (Figure 1) (p < 0.001).
LDH Enzyme Activity Assay
The activity of the LDH enzyme demonstrated a significant increase in both cell lines treated with melatonin and naringin. As shown in Figure 2, melatonin treatment led to a marked elevation in LDH activity in the SKBR3 cell line compared to the control group. Similarly, in the MCF-7 cell line, naringin treatment resulted in a significant increase in LDH activity (p < 0.001). The data also indicated that the LDH enzyme levels were significantly higher in the treated MCF-7 cells compared to the untreated group. Furthermore, an increase in LDH activity was observed in the SKBR3 cell line treated with naringin, highlighting the cytotoxic impact of both compounds on these breast cancer cells.
MDA Levels and Lipid Peroxidation Assay
The levels of malondialdehyde (MDA), a common marker of oxidative stress, were assessed under various treatment conditions. The results indicated a significant increase in MDA levels in MCF-7 cells treated with naringin compared to the control group. Similarly, in SKBR3 cells, naringin treatment led to a marked rise in MDA levels (p < 0.001). Additionally, lipid peroxidation assays revealed that melatonin treatment caused a considerable increase in MDA levels in the SKBR3 and MCF-7 cell lines compared to the control group. This significant elevation of MDA in both cell lines treated with melatonin and naringin underscores the oxidative stress induced by these compounds, contributing to their cytotoxic effects on breast cancer cells (Figure 3) (p < 0.001).
SOD Enzyme Activity Assay
The results indicated a significant decrease in SOD enzyme activity in the MCF-7 cell line treated with naringin and melatonin compared to the control group (p < 0.001), as shown in Figure 4. This reduction in SOD activity highlights the impact of melatonin and naringin on modulating oxidative stress in these SKBR3 and MCF-7 breast cancer cells.
Comparison of Mechanisms Overlapping Mechanisms
Both melatonin and naringin induce oxidative stress (evidenced by increased MDA levels) and cytotoxicity (evidenced by increased LDH activity) in breast cancer cells. Both compounds also result in a reduction of SOD activity, indicating a compromised antioxidant defense. Distinct Mechanisms: While both compounds show similar effects in terms of oxidative stress and cytotoxicity, the extent of these effects might vary between different cell lines (SKBR3 and MCF-7). Additionally, the specific pathways and molecular targets leading to these outcomes could differ between melatonin and naringin, which would require further investigation. This analysis highlights that both melatonin and naringin have overlapping mechanisms in inducing oxidative stress and cytotoxicity in breast cancer cells, yet their distinct pathways and targets could offer unique therapeutic insights.
Discussion
The present study highlights the promising anticancer properties of naringin and melatonin in breast cancer treatment, focusing on two specific cell lines, SKBR3 (HER2+) and MCF-7 (HER2-). The findings reveal significant insights into how these compounds may contribute to cancer therapy by inducing cell death, reducing oxidative stress, and modulating antioxidant enzyme activity.
Cytotoxic Effects of Naringin and Melatonin
The results from the MTT assay demonstrated that both naringin and melatonin exhibit dose-dependent cytotoxic effects on SKBR3 and MCF-7 cells. The increased cell death observed with higher concentrations of naringin underscores its potential as an effective anticancer agent. Notably, the SKBR3 cell line showed a more pronounced response to naringin treatment compared to the MCF-7 cell line, suggesting a differential sensitivity, which could be attributed to the distinct molecular characteristics of these cell lines.
Previous studies have also reported the cytotoxic effects of naringenin on various cancer cell lines. A systematic review by A. Rauf et al. (2022) highlighted the potential of naringin in reducing tumor growth and inducing apoptosis in cancer cells8. Additionally, Li et al. (2016) reported that naringin inhibited the growth of MCF-7 breast cancer cells by inducing cell cycle arrest and apoptosis9. Similarly, Talib et al. (2022) reviewed the role of melatonin in cancer treatment and suggested its potential to enhance the efficacy of conventional therapies10. Moreover, Gao et al. (2020) demonstrated that melatonin suppressed the proliferation of human breast cancer cells and sensitized them to chemotherapy11.
The differential sensitivity of SKBR3 (HER2+) and MCF-7 (HER2-) cells to naringin and melatonin treatment may be attributed to the distinct molecular characteristics of each cell line. In SKBR3 cells, the overexpression of the HER2 receptor leads to the activation of downstream PI3K/AKT and MAPK signaling pathways, promoting cell proliferation and survival. Naringin and melatonin might inhibit HER2-mediated signaling, thereby reducing cell viability and inducing apoptosis through the disruption of these pathways. Additionally, their ability to modulate oxidative stress could further sensitize HER2+ cells to treatment12, 13, 14, 15.
In contrast, MCF-7 cells, which rely on estrogen receptor (ER) signaling, might respond to naringin and melatonin through different mechanisms. Naringin has been shown to downregulate ERα expression, while melatonin exhibits anti-estrogenic properties, both leading to reduced cell proliferation and increased apoptosis in ER-positive cells. The differential impact on antioxidant enzyme activities and lipid peroxidation levels between the two cell lines could also play a role in their varying sensitivity to these compounds16, 17.
Oxidative Stress and Antioxidant Response
The study further explored the oxidative stress response by measuring MDA levels, a common marker of lipid peroxidation. A significant increase in MDA levels in both SKBR3 and MCF-7 cells treated with naringin indicates enhanced oxidative stress, which likely contributes to the observed cytotoxicity. The elevated MDA levels correlate with increased cell death, reinforcing the role of oxidative stress in the anticancer effects of naringin18, 19.
Previous research has shown that naringin can induce oxidative stress in cancer cells, leading to cell death. For example, Zhang et al. (2017) found that naringin induced oxidative stress and apoptosis in human lung cancer cells20. Additionally, M. Hassan et al. (2023) demonstrated that melatonin inhibits the NF-κB signaling pathway, reducing tumor size and growth in MDA-MB-231 cell line xenografts17. This aligns with our findings, suggesting that both naringin and melatonin can modulate oxidative stress and enhance cancer cell death. Furthermore, Reiter et al. (2017) reviewed melatonin’s role in reducing oxidative stress in cancer cells and highlighted its potential to improve cancer therapy outcomes21.
Enzyme Activity Modulation
The activity of key antioxidant enzymes, LDH and SOD, was also assessed. The significant increase in LDH activity in naringin-treated MCF-7 and SKBR3 cells suggests heightened cellular damage and membrane permeability. In contrast, melatonin treatment resulted in a notable inhibition of SOD activity in MCF-7 cells, indicating its potential to modulate antioxidant defenses and reduce oxidative stress22. The differential enzyme activity patterns between the two compounds highlight their unique mechanisms of action in combating cancer cells.
Our findings are consistent with prior studies that reported similar enzyme activity modulation. For instance, Khaled et al. (2022) found that naringin increased LDH levels in breast cancer cells, indicating cellular damage23. Moreover, Reiter et al. (2024) demonstrated that melatonin reduced SOD activity, thereby enhancing the oxidative stress-mediated apoptosis in cancer cells24.
Implications for Breast Cancer Therapy
These findings collectively suggest that naringin and melatonin hold significant promise as complementary agents in breast cancer therapy. Naringin’s ability to induce oxidative stress and cell death, coupled with melatonin’s modulation of antioxidant defenses, points to a synergistic potential that warrants further investigation. The distinct responses of SKBR3 and MCF-7 cells to these treatments underscore the importance of considering molecular subtype-specific strategies in cancer therapy.
Further research is essential to isolate and characterize the active compounds within naringin and melatonin and to evaluate their efficacy in vivo. Conducting in vivo studies would provide valuable insights into the pharmacokinetics, pharmacodynamics, and potential therapeutic benefits of these compounds in a more physiologically relevant context. Additionally, understanding the molecular pathways influenced by these compounds could provide deeper insights into their mechanisms of action and potential combinatory effects. Exploring the synergistic potential of naringin and melatonin in combination with established therapies, such as chemotherapy or targeted treatments, could enhance their translational relevance and therapeutic efficacy. The exploration of naringin and melatonin in clinical settings, particularly in combination therapy regimens, could pave the way for novel therapeutic approaches, improving outcomes for breast cancer patients.
Conclusion
The present study underscores the significant anticancer potential of naringin and melatonin in treating breast cancer, as evidenced by their effects on SKBR3 (HER2+) and MCF-7 (HER2-) cell lines. Both compounds demonstrated dose-dependent cytotoxicity, with higher concentrations leading to increased cell death. Naringin showed a pronounced effect on SKBR3 cells, indicating its effectiveness in targeting distinct cancer cell characteristics. Additionally, both naringin and melatonin were found to modulate oxidative stress markers, with naringin increasing MDA levels and LDH activity, while melatonin decreased SOD activity, reinforcing their roles in inducing oxidative stress.
These findings align with prior research, highlighting the cytotoxic and apoptotic effects of naringin and melatonin on various cancer cells. The modulation of key antioxidant enzymes further supports their potential to enhance cancer cell apoptosis through oxidative stress pathways. Consequently, naringin and melatonin present promising complementary agents in breast cancer therapy.
Abbreviations
ANOVA: Analysis of Variance, DGK: German Biochemical Society, DMEM: Dulbecco's Modified Eagle Medium, EDTA: Ethylenediaminetetraacetic acid, ELISA: Enzyme-Linked Immunosorbent Assay, FBS: Fetal Bovine Serum, HER2: Human Epidermal growth factor Receptor 2, IC50: Inhibitory Concentration 50%, IL: Illinois (as part of Chicago, IL, USA), LDH: Lactate Dehydrogenase, MDA: Malondialdehyde, MCF-7: Name of a breast cancer cell line, MAPK: Mitogen-Activated Protein Kinase, MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, NADH: Nicotinamide Adenine Dinucleotide + Hydrogen, NF-κB: Nuclear Factor kappa-light-chain-enhancer of activated B cells, NBT: Nitro Blue Tetrazolium, PI3K/AKT: Phosphoinositide 3-kinase/Protein kinase B, SD: Standard Deviation, SKBR3: Name of a breast cancer cell line, SOD: Superoxide Dismutase, SPSS: Statistical Package for the Social Sciences
Acknowledgments
None.
Author’s contributions
All authors contributed to the study conception and design. data collection, and analysis were performed by all authors. All authors read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Wilkinson
L.,
Gathani
T.,
Understanding breast cancer as a global health concern. The British Journal of Radiology.
2022;
95
(1130)
:
20211033
.
View Article PubMed Google Scholar -
Ghanbari-Movahed
M.,
Jackson
G.,
Farzaei
M.H.,
Bishayee
A.,
A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Frontiers in Pharmacology.
2021;
12
:
639840
.
View Article PubMed Google Scholar -
Dehelean
C.A.,
Marcovici
I.,
Soica
C.,
Mioc
M.,
Coricovac
D.,
Iurciuc
S.,
Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules (Basel, Switzerland).
2021;
26
(4)
:
1109
.
View Article PubMed Google Scholar -
Blask
D.E.,
Melatonin in oncology. In MelatoninCRC Press 2020.
View Article Google Scholar -
Menéndez-Menéndez
J.,
Martínez-Campa
C.,
Melatonin: an anti-tumor agent in hormone-dependent cancers. International Journal of Endocrinology.
2018;
2018
(1)
:
3271948
.
View Article PubMed Google Scholar -
Alam
M.A.,
Subhan
N.,
Rahman
M.M.,
Uddin
S.J.,
Reza
H.M.,
Sarker
S.D.,
Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Advances in Nutrition.
2014;
5
(4)
:
404-17
.
View Article PubMed Google Scholar -
Kumar
P.,
Nagarajan
A.,
Uchil
P.D.,
Analysis of cell viability by the MTT assay. Cold spring harbor protocols.
2018;
2018
(6)
:
pdb. prot095505
.
View Article Google Scholar -
Rauf
A.,
Shariati
M.A.,
Imran
M.,
Bashir
K.,
Khan
S.A.,
Mitra
S.,
Comprehensive review on naringenin and naringin polyphenols as a potent anticancer agent. Environmental Science and Pollution Research International.
2022;
29
(21)
:
31025-41
.
View Article PubMed Google Scholar -
Li
N.,
Whitaker
C.,
Xu
Z.,
Heggeness
M.,
Yang
S.Y.,
Therapeutic effects of naringin on degenerative human nucleus pulposus cells for discogenic low back pain. The Spine Journal.
2016;
16
(10)
:
1231-7
.
View Article PubMed Google Scholar -
Talib
W.H.,
Rahim
S.A.,
Al-Shdifat
L.M.,
Mahmod
A.I.,
Chapter 14: Melatonin in cancer research and treatment: From bench to bedside. In Melatonin 2024.
View Article Google Scholar -
Gao
J.,
Su
G.,
Liu
J.,
Zhang
J.,
Zhou
J.,
Liu
X.,
Mechanisms of inhibition of excessive microglial activation by melatonin. Journal of Molecular Neuroscience.
2020;
70
(8)
:
1229-36
.
View Article PubMed Google Scholar -
Shayan
S.,
Osgoei
L.T.,
Jouni
F.J.,
Effect of capecitabine and melatonin on HER2+ (SK-BR-3) and HER2-(MCF7) human breast cancer cell lines. Tropical Journal of Pharmaceutical Research.
2023;
22
(7)
:
1387-93
.
View Article Google Scholar -
Obeagu
E.I.,
Babar
Q.,
Vincent
C.,
Udenze
C.L.,
Eze
R.,
Okafor
C.J.,
Therapeutic targets in breast cancer signaling: a review. Journal of Pharmaceutical Research International.
2021;
33
:
82-99
.
View Article Google Scholar -
Nagaprashantha
L.D.,
Singhal
J.,
Chikara
S.,
Gugiu
G.,
Horne
D.,
Awasthi
S.,
2'-Hydroxyflavanone induced changes in the proteomic profile of breast cancer cells. Journal of Proteomics.
2019;
192
:
233-45
.
View Article PubMed Google Scholar -
Golbashirzadeh
M.,
Heidari
H.R.,
Aghamolayi
A.A.,
Fattahi
Y.,
Talebi
M.,
Khosroushahi
A.Y.,
In vitro siRNA-mediated GPX4 and AKT1 silencing in oxaliplatin resistance cancer cells induces ferroptosis and apoptosis. Medical Oncology (Northwood, London, England).
2023;
40
(10)
:
279
.
View Article PubMed Google Scholar -
Xu
Z.,
Huang
B.,
Liu
J.,
Wu
X.,
Luo
N.,
Wang
X.,
Combinatorial anti-proliferative effects of tamoxifen and naringenin: the role of four estrogen receptor subtypes. Toxicology.
2018;
410
:
231-46
.
View Article PubMed Google Scholar -
Hasan
M.,
Browne
E.,
Guarinoni
L.,
Darveau
T.,
Hilton
K.,
Witt-Enderby
P.A.,
Novel melatonin, estrogen, and progesterone hormone therapy demonstrates anti-cancer actions in MCF-7 and MDA-MB-231 breast cancer cells. Breast Cancer (Auckland).
2020;
14
:
1178223420924634
.
View Article PubMed Google Scholar -
Memariani
Z.,
Abbas
S.Q.,
Hassan
S.S. Ul,
Ahmadi
A.,
Chabra
A.,
Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacological Research.
2021;
171
:
105264
.
View Article PubMed Google Scholar -
Golbashirzadeh
M.,
Heidari
H.R.,
Khosroushahi
A.Y.,
Molecular mechanisms of reactive oxygen species in regulated cell deaths: impact of ferroptosis in cancer therapy. Gene Reports.
2022;
27
:
101614
.
View Article Google Scholar -
Zhang
J.,
Yang
S.,
Li
H.,
Chen
F.,
Shi
J.,
Naringin ameliorates diabetic nephropathy by inhibiting NADPH oxidase 4. European Journal of Pharmacology.
2017;
804
:
1-6
.
View Article PubMed Google Scholar -
Reiter
R.J.,
Rosales-Corral
S.A.,
Tan
D.X.,
Acuna-Castroviejo
D.,
Qin
L.,
Yang
S.F.,
Melatonin, a full service anti-cancer agent: inhibition of initiation, progression and metastasis. International Journal of Molecular Sciences.
2017;
18
(4)
:
843
.
View Article PubMed Google Scholar -
Franco
P.I.,
Neto
J.R. do Carmo,
Milhomem
A.C.,
Machado
J.R.,
Miguel
M.P.,
Antitumor effect of melatonin on breast cancer in experimental models: A systematic review. Biochimica et Biophysica Acta. Reviews on Cancer.
2023;
1878
(1)
:
188838
.
View Article PubMed Google Scholar -
Khaled
S.S.,
Soliman
H.A.,
Abdel-Gabbar
M.,
Ahmed
N.A.,
Attia
K.A.,
Mahran
H.A.,
The Preventive Effects of Naringin and Naringenin against Paclitaxel-Induced Nephrotoxicity and Cardiotoxicity in Male Wistar Rats. Evidence-Based Complementary and Alternative Medicine.
2022;
2022
(1)
:
8739815
.
View Article PubMed Google Scholar -
Reiter
R.J.,
Sharma
R.,
Chuffa
L.G. DA,
Zuccari
D.A.,
Amaral
F.G.,
Cipolla-Neto
J.,
Melatonin-mediated actions and circadian functions that improve implantation, fetal health and pregnancy outcome. Reproductive Toxicology (Elmsford, N.Y.).
2024;
124
:
108534
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 12 No 2 (2025)
Page No.: 7109-7117
Published on: 2025-02-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 1259 times
- PDF downloaded - 415 times
- XML downloaded - 89 times
 Biomedpress
Biomedpress





