Abstract
Introduction: Pleural effusion (PE) is commonly observed in clinical practice. Conventional smear (CS), cell block (CB), and liquid-based cytology (LBC) of pleural fluid are used to guide the diagnosis of malignant pleural effusion (MPE). However, the effectiveness of these cytological techniques, whether used alone or in combination, is still not well established.
Methods: A prospective study was conducted from October 2019 to May 2020 at the Department of Pulmonary Medicine, Cho Ray Hospital (Ho Chi Minh City, Vietnam). Suspected MPE patients were investigated by simultaneous cytological methods (CS, CB, and LBC) of pleural fluid and pleural tissue histopathology. The study included 47 patients with MPE, confirmed by pleural tissue pathology as the gold standard. The study compares the sensitivity of the three cytological methods.
Results: Out of 69 patients suspected of MPE, 47 were confirmed to have MPE through histopathological analysis of pleural biopsy. The average age of the study participants was 62.6 years, with 44.7% being male. The sensitivity of LBC and CB, LBC and CS, and CB and CS did not significantly differ, with p-values of 0.546, 0.789, and 0.606, respectively (McNemar test). Combining two of the three cytological methods (CS, CB, LBC) significantly enhances the sensitivity in diagnosing MPE compared to using a single method alone, except for the combination of CB and LBC versus LBC alone. The study has limitations, including the exclusion of non-MPE pleural effusion cases, which means the specificity of the cytological methods could not be calculated. Additionally, the limited number of patients should be taken into consideration, and caution is advised when interpreting the study results.
Conclusions: The study concludes that CS, CB, and LBC methods have similar sensitivity in diagnosing MPE. Combining any two methods improves diagnostic sensitivity compared to using a single method, although CB and LBC together do not surpass LBC alone. Clinicians should consider combining cytological techniques to optimize the diagnostic sensitivity of MPE.
Introduction
Pleural effusion (PE) is frequently encountered in clinical practice and affects more than 0.3% of the population annually1. Common etiologies of PE include heart failure, malignant pleural effusion (MPE), parapneumonic effusion, and pulmonary embolism2. Identifying malignant cells in pleural fluid is warranted if the initial diagnosis suspects MPE. Pleural fluid cytology testing includes three methods: conventional smear (CS), cellblock (CB), and liquid-based cytology (LBC).
Although LBC was developed later than CS and CB3, 4, numerous investigations have demonstrated that LBC is capable of diagnosing malignant disorders as effectively as, or even better than, other cytological methods in various specimen types, including sputum5, fine-needle aspiration of thyroid lesions6, bronchoalveolar lavage7, transbronchial needle aspiration8, and pleural fluid9, 10. In clinical practice, it is essential to use CS, CB, and LBC of pleural fluid to guide the diagnosis of MPE. However, the diagnostic efficacy remains unclear whether these cytological methods are used individually or in combination. This study aimed to compare the diagnostic value of these cytological methods when performed separately or in combination for diagnosing MPE.
Methods
A prospective study was conducted from October 2019 to May 2020 at the Department of Pulmonary Medicine, Cho Ray Hospital (Ho Chi Minh City, Vietnam). Patients suspected of having MPE were identified based on a history of lung cancer or other malignancies, smoking, and prolonged symptoms over several weeks, such as cough, chest pain, dyspnea, hemoptysis, weight loss, or chest imaging showing PE with a suspicious lung mass. Suspected MPE patients admitted to the hospital were included in the study, and simultaneous cytological methods (CS, CB, and LBC) of pleural fluid and pleural tissue histopathology were performed. Finally, the study included 47 patients with MPE, confirmed by pleural tissue pathology as the gold standard.
Inclusion Criteria
The study included patients fulfilling all of the following criteria: i) aged 18 years or older, ii) consenting to participate in the study, iii) having a confirmed diagnosis of MPE through pleural tissue histopathology obtained via blind pleural biopsy, and iv) undergoing all three cytological methods, including CS, CB, and LBC.
Exclusion Criteria
Patients with the following characteristics were excluded: i) contraindications for blind pleural biopsy, ii) undergoing cancer treatment with chemotherapy, radiotherapy, or targeted therapy, and iii) a known diagnosis of pleural cancer (either primary or metastatic) in their medical history.
Conventional Smear
The pleural fluid was divided into two portions with a minimum volume of 5 mL each. One portion was smeared and stained with methylene blue, then observed under a light microscope to determine the number of red blood cells and nucleated cells. The remaining portion was centrifuged, smeared, and stained with Wright-Giemsa stain, and then observed under a light microscope to identify atypical cells suspected of malignancy.
Cellblock
To perform the CB method, at least 60 mL of pleural fluid should be used. The fluid was allowed to sit for 8-10 hours, after which the supernatant was removed, and the cell sediment was distributed into small tubes. The tubes were centrifuged at 2000 rpm for 10 minutes, the supernatant was discarded, and the sediment was fixed in 10% formalin at 60°C for about 2 hours. After the formalin was discarded, the cell block was removed, wrapped in paper, and placed into a plastic mold. The mold was then processed according to routine histology procedures (paraffin embedding), sections 3-5 µm thick were cut, stained with hematoxylin-eosin, and immunohistochemical staining was performed if needed.
Liquid-Based Cytology
The minimum amount of pleural fluid required to perform the LBC method was set at 20 mL. After the sample was centrifuged, the supernatant was discarded, and the cell components at the bottom were collected. To wash the sample, 30 mL of CytoLyt® solution was added, and another round of centrifugation was conducted. If the cell components were clean and clear, they were transferred to a container with PreservCyt® solution for stabilization. The sample was stabilized for 15 minutes before it was processed using the ThinPrep® 2000 processor (Hologic, USA).
Pleural Tissue Pathology
At least three pleural tissue samples obtained through blind pleural biopsy were fixed in 10% formalin and embedded in paraffin. The specimens were stained with Hematoxylin-Eosin. Immunohistochemical staining was performed if needed to differentiate malignant cells from mesothelial cells.
Cytological and Histopathological Report
Pleural fluid CS results were interpreted by a cytologist with over 5 years of experience and were reported as positive if abnormal or suspicious malignant cells were detected. Pleural fluid CB results were interpreted by a pathologist with over 3 years of experience and were reported as positive if abnormal, suspicious malignant, or cancer cells were detected. Additionally, pleural fluid LBC results were also read by a pathologist with over 3 years of experience, with positive results reported if abnormal or suspicious malignant cells were identified. All physicians interpreting the cytological and histopathological results were completely blinded to the clinical information and the results of other methods.
Statistical Analysis
The statistical analysis was performed using Stata version 14 (StataCorp). Quantitative variables with a normal distribution were represented as mean ± standard deviation (SD), whereas those with a non-normal distribution were shown as medians. Qualitative variables were expressed as percentages. The sensitivity of CS, CB, and LBC was calculated. The McNemar test was used to compare paired data proportions in the same population. A p-value of less than 0.05 was considered statistically significant.
Results
Patient Characteristics
We enrolled 69 patients suspected of having MPE, of whom 47 were definitively diagnosed with MPE based on histopathological examination of pleural biopsy specimens for inclusion in the final analysis. The study population had a mean age of 62.6 years, with 44.7% being male. Detailed information is shown in Table 1.
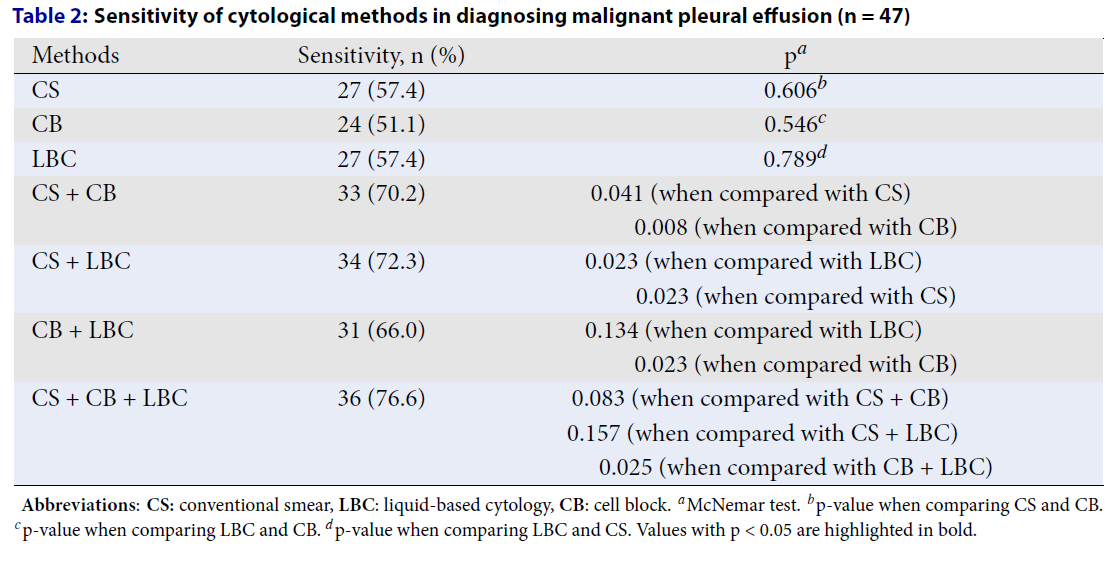
The Value of Cytological Methods
Discussion
Our study simultaneously applied three cytological methods to the pleural fluid specimen to compare their sensitivity in detecting cancer cells against the gold standard of histopathological examination. The results indicate that combining two of the three cytological methods (CS, CB, LBC) significantly improves the sensitivity in diagnosing MPE compared to using a single method alone (except for the combination of CB + LBC compared to LBC alone).
There are several mechanisms leading to PE associated with malignancies, which can be categorized into four main groups: PE due to the local effects of the tumor (e.g., lymphatic obstruction, atelectasis from bronchial obstruction), PE due to the systemic effects of the tumor (e.g., pulmonary embolism, hypoalbuminemia), and PE as a complication of radiation or chemotherapy (e.g., methotrexate, cyclophosphamide)11. However, not all cases of PE in malignancies involve the presence of cancer cells in the pleural fluid or on the pleural surface. The authors suggest that the primary mechanism leading to MPE is the tumor's increased secretion of vascular endothelial growth factor (VEGF) and the resultant increased vascular permeability12, 13. In cases where lung cancer metastasizes to the pleura, it is classified as M1 in the TNM (tumor, node, metastasis) staging system, which negatively impacts prognosis. Therefore, identifying pleural cancer is crucial for determining the treatment strategy and prognosis for the patient14. In practice, pleural fluid cytology testing demonstrates higher sensitivity compared to pleural tissue histopathology obtained through blind pleural biopsy. This is due to several factors: a) approximately 50% of pleural metastatic cancers do not involve the parietal pleura15, b) the biopsy is conducted at a single site, and c) the biopsy is performed blindly11. Thus, enhancing the sensitivity of pleural fluid cytology through the combination of methods such as CS, CB, and LBC can improve diagnostic accuracy. This approach may reduce the need for blind pleural biopsy, an inherently more invasive procedure.
Unlike CS and CB methods, which have been widely applied in medical practice, LBC is a more recent development. However, numerous studies on various types of specimens have shown that LBC may offer superior diagnostic capabilities compared to CS and CB8, 10, 16, 17. LBC offers several advantages, including the creation of a thin, uniform layer of cells without overlapping or obscuring factors such as artifacts or blood and preserving cellular morphology. Additionally, LBC provides representative and incidental sampling, which helps reduce the time required for result interpretation18, 19. Our study demonstrates that the sensitivity of CS, CB, and LBC in diagnosing MPE does not show a statistically significant difference. However, combining two of the three cytological methods significantly improves sensitivity in diagnosing MPE compared to using a single method alone (except for the combination of CB + LBC compared to LBC alone). The advantages of LBC mentioned above, along with the findings of this study, which demonstrate that LBC has higher sensitivity than CB and that the combination of CB and LBC does not further increase sensitivity compared to LBC alone, suggest the potential superiority of LBC over CB in diagnosing MPE.
| Variables | Normal value | n (%) | Mean ± SD |
|---|---|---|---|
| Age (years) | 62.6 ± 12.7 | ||
| Male | 21 (44.7) | ||
| Female | 26 (55.3) | ||
| History of cancer | |||
| Lung cancer | 7 (14.9) | ||
| Non-lung cancer | 2 (4.3) | ||
| Symptom | |||
| Fever | 0 (0.0) | ||
| Dyspnea | 35 (74.5) | ||
| Cough | 28 (59.6) | ||
| Chest pain | 27 (57.4) | ||
| Hemotypsis | 3 (6.4) | ||
| Weight loss | 7 (14.9) | ||
| Location of pleural effusion on chest X-ray | |||
| Right side | 19 (40.4) | ||
| Left side | 21 (44.7) | ||
| Both sides | 7 (14.9) | ||
| Cellular components of pleural fluid | |||
| Lymphocyte > 50% | 30 (63.8) | ||
| Eosinophil > 10% | 2 (4.3) | ||
| Serum total protein (g/dL) | 6–8 | 6.1 [5.7–6.6] | |
| Pleural fluid protein (g/dL) | <1.5 | 4.2 [3.7–4.8] | |
| Serum LDH (U/L) | 140–280 | 266 [216–387] | |
| Pleural fluid LDH (U/L) | 638 [327–1084] | ||
| Pleural fluid ADA (U/L) | 9.9 [6.2–13.5] | ||
| Exudates pleural effusion bases on Light’s criteria, n (%) | 47 (100%) | ||
| Methods | Sensitivity, n (%) | p a |
|---|---|---|
| CS | 27 (57.4) | 0.606 b |
| CB | 24 (51.1) | 0.546 c |
| LBC | 27 (57.4) | 0.789 d |
| CS + CB | 33 (70.2) | 0.041 (when compared with CS) 0.008 (when compared with CB) |
| CS + LBC | 34 (72.3) | 0.023 (when compared with LBC) 0.023 (when compared with CS) |
| CB + LBC | 31 (66.0) | 0.134 (when compared with LBC) 0.023 (when compared with CB) |
| CS + CB + LBC | 36 (76.6) | 0.083 (when compared with CS + CB) 0.157 (when compared with CS + LBC) 0.025 (when compared with CB + LBC) |
A study involving 278 patients with MPE compared the sensitivity of LBC and CB methods in diagnosing MPE. The study found that the sensitivity of LBC and CB was 61.2% and 61.9%, respectively, with no statistically significant difference (p = 0.89), similar to the results of our study. The specimen processing was performed using the ThinPrep® 2000 processor (Hologic). However, the simultaneous combination of both LBC and CB methods significantly improved the diagnostic sensitivity compared to using each method alone10. In a 2018 study by Woo involving 862 patients with PE, the sensitivity of LBC, CB, and the combination of LBC and CB were 81.3%, 94.3%, and 98.3%, respectively. The specimen processing was performed using the CellprepPlus® processor (Biodyne, South Korea)20. The sensitivity of LBC and CB in our study is comparable to that reported by Assawasaksakul (57.4% vs. 61.2% for LBC and 51.1% vs. 61.9% for CB), with both studies utilizing the same LBC technique (ThinPrep® 2000 processor, Hologic)10. However, Woo's study reports significantly higher sensitivity for both LBC and CB, which can be attributed to differences in study design. Woo's study diagnosed MPE using cytology as the gold standard, whereas our study used pleural biopsy pathology as the gold standard. Additionally, variations in the LBC processing equipment may also contribute to these differences in sensitivity20. Another study has also shown no significant difference between modified LBC and CytoRich Red preservatives in diagnosing malignant effusion, similar to the results of our study21.
Combining all three cytological methods shows the highest sensitivity in diagnosing MPE, with a sensitivity of 76.6%. However, this higher sensitivity is not statistically significant when compared to the use of only CS combined with CB or CS combined with LBC. The simultaneous use of all cytological methods may aid increase the detection of cancer cells in pleural fluid, which can explain the highest sensitivity observed when combining all three methods. However, due to the small sample size in our study, the difference is not yet clear.
Our study has several limitations. Firstly, we did not determine the origin of the cancer cells present in the pleura, which restricts our ability to assess the impact of different types of cancer on the sensitivity of cytological methods22. Secondly, we did not analyze the correlation between the volume of pleural fluid used in CB and LBC. Previous research has indicated that the diagnostic accuracy of LBC for bronchoalveolar lavage fluid improves with an increased volume of fluid tested7. Thirdly, since this study was conducted at a single center with a small sample size and without calculating the minimum sample size or performing a power analysis, the results of this study should be applied with caution, and selection bias may occur. Finally, our study did not include cases with diagnoses of PE other than MPE, thus limiting our ability to calculate the specificity of each method.
Conclusions
All three cytological methods for pleural fluid—CS, CB, and LBC—demonstrate comparable efficacy in diagnosing MPE. However, combining any two of these methods significantly enhances sensitivity for diagnosing MPE compared to using a single method, except for the CB and LBC combination, which does not show improved sensitivity over LBC alone. This underscores the benefit of using multiple cytological methods to enhance diagnostic accuracy. Clinicians should consider combining cytological techniques to optimize the diagnostic sensitivity for MPE while also taking into account factors such as processing time, test costs, and the expertise available at each medical facility.
Abbreviations
ADA: Adenosine Deaminase, CB: Cell Block, CS: Conventional Smear, IQR: Interquartile Range, LBC: Liquid-Based Cytology, LDH: Lactate Dehydrogenase, MPE: Malignant Pleural Effusion, PE: Pleural Effusion, SD: Standard Deviation
Acknowledgments
None
Author’s contributions
NVH, NTLP, KND, NDM: Conceptualization, Methodology, Writing original draft preparation; NDM, NTN, TDV, DLQ: Visualization, Methodology, Software; KND, NDM, NTN: Data curation; NVH, NTLP, KND, NDM, NTN, TDV: Validation, Investigation; TDV, DLQ: Supervision. All authors read and approved the final manuscript.
Funding
None
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The present study obtained approval from the Ethical Review Committee, University of Medicine and Pharmacy at Ho Chi Minh City (No. 19458/HĐĐĐ-ĐHYD). All patients participating in the study signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Maskell
N.,
Group
undefined British Thoracic Society Pleural Disease Guideline,
British Thoracic Society Pleural Disease Guidelines-2010 update. Thorax.
2010;
65
(8)
:
667-9
.
View Article PubMed Google Scholar -
Marel
M.,
Zr˚ustová
M.,
Stasný
B.,
Light
R.W.,
The incidence of pleural effusion in a well-defined region. Epidemiologic study in central Bohemia. Chest.
1993;
104
(5)
:
1486-9
.
View Article PubMed Google Scholar -
Lee
Y.M.,
Hwang
J.Y.,
Son
S.M.,
Choi
S.Y.,
Lee
H.C.,
Kim
E.J.,
Comparison of diagnostic accuracy between CellprepPlus® and ThinPrep® liquid-based preparations in effusion cytology. Diagnostic Cytopathology.
2014;
42
(5)
:
384-90
.
View Article PubMed Google Scholar -
Asciak
R.,
Rahman
N.M.,
Malignant Pleural Effusion: From Diagnostics to Therapeutics. Clinics in Chest Medicine.
2018;
39
(1)
:
181-93
.
View Article PubMed Google Scholar -
Choi
Y.D.,
Han
C.W.,
Kim
J.H.,
Oh
I.J.,
Lee
J.S.,
Nam
J.H.,
Effectiveness of sputum cytology using ThinPrep method for evaluation of lung cancer. Diagnostic Cytopathology.
2008;
36
(3)
:
167-71
.
View Article PubMed Google Scholar -
Saleh
H.A.,
Hammoud
J.,
Zakaria
R.,
Khan
A.Z.,
Comparison of Thin-Prep and cell block preparation for the evaluation of Thyroid epithelial lesions on fine needle aspiration biopsy. CytoJournal.
2008;
5
(1)
:
3
.
View Article PubMed Google Scholar -
Nguyen-Dang
K.,
Bui-Thi
H.D.,
Duong-Minh
N.,
Pham-Quang
T.,
Nguyen-Ho
L.,
Lam-Quoc
D.,
The Role and Associated Factors of Liquid-Based Cytology of Bronchoalveolar Lavage Fluid in Lung Cancer Diagnosis: A Prospective Study. Cureus.
2023;
15
(11)
:
e48483
.
View Article PubMed Google Scholar -
Nalwa
A.,
Walia
R.,
Singh
V.,
Madan
K.,
Mathur
S.,
Iyer
V.,
Comparison of Conventional Smear and Liquid-based Cytology Preparation in Diagnosis of Lung Cancer by Bronchial Wash and Transbronchial Needle Aspiration. Journal of Cytology / Indian Academy of Cytologists.
2018;
35
(2)
:
94-8
.
View Article PubMed Google Scholar -
Elsheikh
T.M.,
Kirkpatrick
J.L.,
Wu
H.H.,
Comparison of ThinPrep and cytospin preparations in the evaluation of exfoliative cytology specimens. Cancer.
2006;
108
(3)
:
144-9
.
View Article PubMed Google Scholar -
Assawasaksakul
T.,
Boonsarngsuk
V.,
Incharoen
P.,
A comparative study of conventional cytology and cell block method in the diagnosis of pleural effusion. Journal of Thoracic Disease.
2017;
9
(9)
:
3161-7
.
View Article PubMed Google Scholar -
Steven
A.S.,
John
T.H.,
Malignant Pleural Effusion. In: Michael A. G, editor. Fishman's Pulmonary Diseases and Disorders. New York: McGraw-Hill Education; 2015. p. 1188-96.
.
-
Ishimoto
O.,
Saijo
Y.,
Narumi
K.,
Kimura
Y.,
Ebina
M.,
Matsubara
N.,
High level of vascular endothelial growth factor in hemorrhagic pleural effusion of cancer. Oncology.
2002;
63
(1)
:
70-5
.
View Article PubMed Google Scholar -
Cheng
D.,
Rodriguez
R.M.,
Perkett
E.A.,
Rogers
J.,
Bienvenu
G.,
Lappalainen
U.,
Vascular endothelial growth factor in pleural fluid. Chest.
1999;
116
(3)
:
760-5
.
View Article PubMed Google Scholar -
Lababede
O.,
Meziane
M.A.,
The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist.
2018;
23
(7)
:
844-8
.
View Article PubMed Google Scholar -
Canto
A.,
Rivas
J.,
Saumench
J.,
Morera
R.,
Moya
J.,
Points to consider when choosing a biopsy method in cases of pleurisy of unknown origin. Chest.
1983;
84
(2)
:
176-9
.
View Article PubMed Google Scholar -
Yang
Y.,
Zhang
X.,
Lu
J.,
Zarogoulidis
P.,
Wang
X.,
Huang
H.,
Application of liquid-based cytology test of bronchial lavage fluid in lung cancer diagnosis. Thoracic Cancer.
2013;
4
(3)
:
318-22
.
View Article PubMed Google Scholar -
Han
S.,
Yang
W.,
Li
H.,
A study of the application of fiberoptic bronchoscopy combined with liquid-based cytology test in the early diagnosis of lung cancer. Oncology Letters.
2018;
16
(5)
:
5807-12
.
View Article PubMed Google Scholar -
Kurtycz
D.F.,
Hoerl
H.D.,
Thin-layer technology: tempered enthusiasm. Diagnostic Cytopathology.
2000;
23
(1)
:
1-5
.
View Article PubMed Google Scholar -
Wu
G.P.,
Wang
E.H.,
Li
J.H.,
Fu
Z.M.,
Han
S.,
Clinical application of the liquid-based cytological test in cytological screening of sputum for the diagnosis of lung cancer. Respirology (Carlton, Vic.).
2009;
14
(1)
:
124-8
.
View Article PubMed Google Scholar -
Woo
C.G.,
Son
S.M.,
Han
H.S.,
Lee
K.H.,
Choe
K.H.,
An
J.Y.,
Diagnostic benefits of the combined use of liquid-based cytology, cell block, and carcinoembryonic antigen immunocytochemistry in malignant pleural effusion. Journal of Thoracic Disease.
2018;
10
(8)
:
4931-9
.
View Article PubMed Google Scholar -
Jangsiriwitayakorn
P.,
Patarapadungkit
N.,
Chaiwiriyakul
S.,
Thongbor
R.,
Sirivech
P.,
Nititarakul
L.,
Comparative Analysis of Modified Liquid-Based Cytology and CytoRich Red Preparation in Assessment of Serous Effusion for Cancer Diagnosis. Asian Pacific Journal of Cancer Prevention.
2018;
19
(6)
:
1571-5
.
View Article PubMed Google Scholar -
Pairman
L.,
Beckert
L.E.,
Dagger
M.,
Maze
M.J.,
Evaluation of pleural fluid cytology for the diagnosis of malignant pleural effusion: a retrospective cohort study. Internal Medicine Journal.
2022;
52
(7)
:
1154-9
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 12 No 2 (2025)
Page No.: 7125-7130
Published on: 2025-02-28
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 1267 times
- PDF downloaded - 437 times
- XML downloaded - 71 times
 Biomedpress
Biomedpress


