Abstract
Introduction: Statins are frequently prescribed for patients with hyperlipidemia to prevent cardiovascular disease, however, there is an inter-individual variability in their efficacy due to many factors, including genetic polymorphism. This study aimed to determine the association between single nucleotide polymorphism (SNP) in the gene cluster SORT1-CELSR2-PSRC1 (rs646776) and lipid profiles in a subset of statin users in Malaysia.
Methods: In total, 122 statin-treated patients were recruited in this cross-sectional study. Genomic DNA from whole blood samples (3 mL) was extracted and genotyped using amplification-refractory mutation system-polymerase chain reaction (ARMS-PCR) for the rs646776 polymorphism. The association between the SNP and statin-related lipid profile changes was evaluated using a dominant genetic model (AA vs. GA + GG genotypes).
Results: The minor allele frequency of the rs646776 was 0.08 and the allele frequency of each genotype was in the Hardy–Weinberg equilibrium (P = 0.6149). Variant allele carriers of rs646776 showed higher (P < 0.05) high-density lipoprotein cholesterol (HDL-C) levels after statin treatment in females but not in males. Conversely, AA genotypes were linked to a significant decrease in total cholesterol and low-density lipoprotein cholesterol (P < 0.01).
Conclusion: Our study provides the first frequency data for PSRC1/CELSR2/SORT1 rs646776 in the Southeast Asian region and further confirms the SNP association with improved HDL-C levels, especially in females using statins. The findings warrant further replication studies to validate the SNP association among different ethnicities in Asia.
Introduction
Statin, a cholesterol-lowering drug, is commonly used to prevent cardiovascular disease because it is highly effective at lowering cholesterol levels. However, statin efficacy has an inter-individual variability on lipid profiles which can be partially caused by genetic variation. As indicated by a genome-wide association study (GWAS) on Europeans and South Asians, the SORT1/CELSR2/PSRC1 rs646776 polymorphism has a strong association with plasma lipoproteins, hence the increased risk of coronary artery disease (CAD)1. In brief, rs646776 is a single nucleotide polymorphism (SNP) on chromosome 1 (i.e., 1p13.3) which resides in the intergenic region of three distinct genes: CELSR2, PSRC1, and SORT12. Minor allele carriers of the SNP are associated with decreased cardiovascular disease risk since it has been shown to lower circulating cholesterol levels, particularly low-density lipoprotein cholesterol (LDL-C), among statin users3. Similarly, another GWAS found that rs646776 was associated with significantly larger LDL-C reductions of up to 0.47%4.
Evidence from animal and in vitro studies showed that SORT1, out of the three candidates in the gene cluster, was the main regulator of lipoprotein metabolism, especially of LDL-C. However, there was no unidirectional evidence for the effect of SORT1 expression on plasma lipid profiles5. In fact, a large meta-analysis from a GWAS on Europeans has associated SORT1 rs646776, particularly owing to its direct involvement in lipid metabolism, with an enhanced statin-related LDL-C response from interacting with apoB at the Golgi apparatus to stimulate LDL-C uptake6. Therefore, we speculate that individuals who inherit these genetic variants have improved lipid profiles, most likely in terms of reduced LDL-C or increased high-density lipoprotein cholesterol (HDL-C) levels, and hence a lower risk of CAD.
Although statins are well-known for their ability to reduce cardiovascular morbidity, an individual's response to a given drug can be influenced by several factors. Besides genetics, the demographic profile of the subject (i.e., age, body mass index, gender) and clinical factors determine the inter-individual variability of statin efficacy7. This study aimed to determine the association between the SORT1/CELSR2/PSRC1 rs646776 polymorphism and statin-related lipid profile changes in a subset of the Malaysian population. Stratification according to the subject’s gender allowed us to evaluate the gene–gender effects on statin-affected lipid profiles. To our knowledge, this study provides the first frequency data for the SNP of Southeast Asian origin.
Methods
Study subjects
After obtaining informed written consent, subjects diagnosed with hyperlipidemia and on statin medication were recruited from the Klinik Rawatan Keluarga at Hospital Universiti Sains Malaysia (HUSM) between May 2018 and October 2020. The recruited subjects included individuals aged between 18 and 75 who had been diagnosed with hyperlipidemia and prescribed statins for at least six weeks. The patients’ medical records were obtained from their clinic files and the hospital’s database. The exclusion criteria are as follows: patients who have been (i) prescribed other lipid-lowering medications as concurrent treatment; (ii) diagnosed with malignancy, liver disease, thyroid disease, kidney disease, or familial hypercholesterolemia; (iii) receiving other medications known to interfere with statin’s efficacy.
SNP genotyping
The DNA from the whole blood (3 mL) was extracted according to the manufacturer’s protocols (GeneAll Biotechnology, Korea). The extracted DNA was then subjected to genotyping using amplification-refractory mutation system-polymerase chain reaction (ARMS-PCR). The temperatures during the ARMS-PCR cycles were as follows: initial denaturation at 95 °C for 5 minutes, 30 denaturation cycles at 94 °C for 30 seconds, annealing at 60.9 °C for 30 seconds, extension at 72 °C for 30 seconds, and an additional extension at 72 °C for 5 minutes. Table 1 lists the primer sets used in this study, which were designed using a website (http://primer1.soton.ac.uk/primer1.html); see Appendix 1 for a supplementary file highlighting the primer sequences within the gene sequences. The products of the ARMS-PCR were visualized on the 2% agarose gel for 60 minutes.
Statistical analysis
The data analysis was carried out using the SPSS software version 26.0 (IBM, United States). The data were reported as frequency (%) and mean ± standard error of the mean (SEM) for categorical and continuous data, respectively. The normality of the continuous data was checked using a test of normality, the Kolmogorov–Smirnov test. For the categorical data, the Chi-square test was used in a contingency table to obtain the odds ratio (OR); the test required at least 80% of the cells to have an expected count greater than 5. Student’s t-test was used to assess differences between two continuous data; in the case of non-parametric data, the Mann–Whitney test was used instead. Repeated measures ANOVA was used to compare means across one or more variables that are based on related subjects; in the case of non-parametric data, the Friedman test was used. When conducting multiple statistical tests, the Bonferroni correction was used to adjust the probability (P) values to avoid a Type I error. The observed genotype frequency was checked for the Hardy–Weinberg equilibrium (HWE) using https://gene-calc.pl/hardy-weinberg-page. Since the homozygous mutant GG genotypes are rare, a genetic dominant model (AA vs. GA + GG genotypes) was applied to assess the SNP association. Multiple binary logistic regression was used to determine which independent factors were associated with the likelihood of achieving the LDL target of < 2.6 mmol/L. Throughout the analysis, a P-value of 0.05 or lower was considered statistically significant.
| Primer sequences | Product size | Interpretation of the genotypes |
|---|---|---|
| FO: 5'-TGGTGAAAAGGACACCTTCC-3' | Outer; 500 bp | Heterozygous; 500 bp, 420 bp and 129 bp |
| FI (G allele): 5'-TATTTGGGAGCAGTGTCATGGACGTG-3' | G allele; 420 bp | Homozygous recessive; 500 bp and 420 bp |
| RI (A allele): 5'-GGCTGATAAGCCTGTCCCTCTGACT -3' | A allele; 129 bp | Wild type; 500 bp and 129 bp |
| RO: 5'-CTTTTCTGCCGTGGATTCTC- 3' |
| Characteristics | n = 122 |
|---|---|
| Age (years + SEM) | 53.62 ± 6.67 |
| Gender, n (%) | |
| Female | 71 (58.2) |
| Male | 51 (41.8) |
| Race, n (%) | |
| Malay | 117 (95.9) |
| Chinese | 3 (2.5) |
| Indian | 1 (0.8) |
| Others | 1 (0.8) |
| Genotype of rs646776, n (%) | |
| a Present study | |
| AA | 102 (83.6) |
| GA | 20 (16.3) |
| GG | 0 |
| Reference population | |
| AA | 461 (91.4) |
| GA | 40 (7.9) |
| GG | 3 (0.5) |
| Type and dosage of statin, n (%) | |
| Atorvastatin | |
| 10 mg/day | 33 (27.0) |
| 20 mg/day | 32 (26.2) |
| 30 mg /day | 4 (3.3) |
| 40 mg /day | 8 (6.6) |
| Simvastatin | |
| 10 mg /day | 4 (3.3) |
| 20 mg /day | 23 (18.9) |
| 40 mg /day | 3 (2.5) |
| Pravastatin | |
| 10 mg /day | 2 (1.6) |
| 20 mg /day | 7 (5.7) |
| Lovastatin | |
| 20 mg /day | 6 (4.9) |
| Comorbidities, n (%) | |
| Hypertension | 52 (42.6) |
| Diabetic + hypertension | 44 (36.0) |
| Diabetic | 12 (9.8) |
| Others | 8 (6.6) |
| None | 6 (4.9) |
| Concomitant drugs, n (%) | |
| Anti-diabetic drugs class | |
| Biguanides only | 1 (0.8) |
| Two> anti-diuretic drugs or more | 11 (9.0) |
| Anti-hypertensive drugs class | |
| Calcium channel blockers only | 17 (13.9) |
| Angiotensin-converting enzyme (ACE) inhibitors only | 5 (4.1) |
| Alpha-blockers only | 1 (0.8) |
| Beta blockers only | 1 (0.8) |
| Two > anti-hypertensive drugs or more | 28 (23.0) |
| Anti-diabetic + Anti-hypertensive | 44 (36.1) |
| Others | 8 (6.6) |
| None | 6 (4.9) |
| Baseline level (mmol/L ± SD) | |
| TC (normal range <5.2 mmol/L) | 5.67 ± 1.56 |
| HDL-C (normal range >1.0 in males and >1.3 in women) | 1.33 ± 0.63 |
| LDL-C (normal range <2.6 mmol/L) | 3.61 ± 1.32 |
| TG (normal range <1.7 mmol/L) | 1.60 ± 0.89 |
| Lipid profile (mmol/L) | AA (n = 102) | GA (n = 20) | P-value |
|---|---|---|---|
| TC (mean ± SD) | |||
| Baseline level | 5.66 ± 1.64 | 5.69 ± 1.03 | 0.936 |
| 0-6 months treatment | 4.61 ± 1.75 | 5.25 ± 0.91 | 0.111 |
| 7-12 months treatment | 5.02 ± 1.58 | 5.11 ± 1.55 | 0.816 |
| a Baseline vs 0-6 months | <.001 | 0.451 | |
| a Baseline vs 7-12 months | 0.004 | 0.344 | |
| HDL-C (mean ± SD) | |||
| Baseline level | 1.32 ± 0.66 | 1.43 ± 0.37 | 0.450 |
| 0-6 months treatment | 1.17 ± 0.41 | 1.42 ± 0.34 | 0.013 |
| 7-12 months treatment | 1.27 ± 0.35 | 1.45 ± 0.38 | 0.043 |
| a Baseline vs 0-6 months | 0.158 | 1.000 | |
| a Baseline vs 7-12 months | 1.000 | 1.000 | |
| LDL-C (mean ± SD) | |||
| Baseline level | 3.68 ± 1.33 | 3.28 ± 1.21 | 0.205 |
| 0-6 months treatment | 2.73 ± 1.27 | 3.11 ± 0.85 | 0.197 |
| 7-12 months treatment | 3.04 ± 1.14 | 3.21 ± 1.02 | 0.539 |
| a Baseline vs 0-6 months | <.001 | 1.000 | |
| a Baseline vs 7-12 months | <.001 | 1.000 | |
| TG (mean ± SD) | |||
| Baseline level | 1.58 ± 0.89 | 1.64 ± 0.91 | 0.783 |
| 0-6 months treatment | 1.48 ± 1.08 | 1.59 ± 0.62 | 0.663 |
| 7-12 months treatment | 1.60 ± 1.09 | 1.38 ± 0.69 | 0.378 |
| a Baseline vs 0-6 months | 0.905 | 1.000 | |
| a Baseline vs 7-12 months | 1.000 | 1.000 |
| Independent variables | P-value | Odds ratio Exp (B) | 95% CI |
|---|---|---|---|
| SORT1/CELSR2/PSRC1 (rs646776) | 0.709 | 1.219 | 0.431 - 3.449 |
| Age | 0.235 | 1.037 | 0.977 - 1.102 |
| a Gender | 0.135 | 0.560 | 0.262 - 1.199 |
| Type of Statin | |||
| Atorvastatin, mg/day | |||
| 10 | 0.725 | 0.000 | 0.000 |
| 20 | 0.934 | 1.045 | 0.365 - 2.998 |
| 30 | 0.112 | 6.900 | 0.637 - 74.690 |
| 40 | 0.695 | 1.380 | 0.275 - 6.921 |
| Simvastatin, mg/day | |||
| 10 | 0.827 | 0.767 | 0.071 - 8.299 |
| S20 | 0.731 | 0.812 | 0.247 - 2.670 |
| S40 | 0.913 | 1.150 | 0.093 - 14.188 |
| Pravastatin, mg/day | |||
| 10 | 0.999 | 0.000 | 0.000 |
| P20 | 0.522 | 1.725 | 0.324 - 9.172 |
| Lovastatin, mg/day | |||
| 20 | 0.106 | 4.600 | 0.721 - 29.332 |
| Baseline lipid (mmol/L) | |||
| Total cholesterol | 0.524 | 0.925 | 0.729 - 1.175 |
| High-density lipoprotein | 0.519 | 0.744 | 0.303 - 1.826 |
| Low-density lipoprotein | 0.688 | 0.941 | 0.699 - 1.267 |
| Triglycerides | 0.389 | 0.820 | 0.522 - 1.289 |
Results
Baseline characteristics of the subjects
A total of 122 subjects with hyperlipidemia, mostly of self-reported Malay ethnicity (95.9%), were recruited into the study. The demographic profiles and clinical characteristics of the subjects are presented in Table 2. The mean age of the subjects is 53 ± 6.67 years; most subjects are female (58.2%) and treated with atorvastatin (63.1%). Many subjects had comorbidities such as diabetes and hypertension, while only 4.9% had no pre-existing diseases. Specifically, 42.6% had hypertension, 36.0% had diabetes and hypertension, 9.8% had diabetes, and the remaining 6.6% had other diseases. Because of their pre-existing illnesses, 88.5% of the subjects were treated with other drugs in addition to statins. Before starting statin therapy, the baseline lipid levels for total cholesterol (TC, 5.67 ± 1.56 mmol/L) and LDL-C (3.61 ± 1.32 mmol/L) were higher than the recommended levels for a healthy individual.
Impact of the SORT1/CELSR2/PSRC1 rs646776 on the lipid profiles of statin users
The lipid levels of the statin users (Table 3) were evaluated over two periods: 0–6 months and 7–12 months. Compared to common AA genotypes, GA genotypes had substantially higher HDL-C levels after 0–6 months (P = 0.013) and 7–12 months (P = 0.043) of statin treatment. Despite not being observed in the GA genotypes, statin treatment resulted in lower TC and LDL-C levels in the AA genotypes after 0–6 months and 7–12 months, both at P < 0.05.
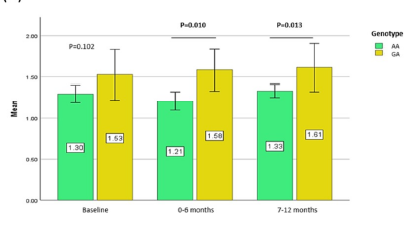
Additionally, we stratified the HDL-C levels based on the patient’s genotypes into males and females to examine the impact of patient gender on the effects of statins on the HDL-C profile (Figure 1). Females with minor allele G carriers (GA) demonstrated significantly higher HDL-C levels after 0–6 months (P = 0.010) and 7–12 months (P=0.013) of statin treatment. Next, multiple binary logistic regression analysis was carried out to determine the association between the independent variables and patient attainment of the target LDL-C level of < 2.6 mmol/L. Table 4 illustrates that none of the variables tested predicted the subject’s attainment of the target LDL-C level of < 2.6 mmol/L.
Discussion
Statins, a lipid-lowering drug, have been used in the primary and secondary prevention of coronary heart disease (CHD). However, considerable heterogeneity in terms of their effects on lipid profiles in a subset of patients may be influenced by interactions between genetics, patient demographics, and other clinical factors8. Our study findings demonstrated a significant association between the SORT1/CELSR2/PSRC1 rs646776 polymorphism and the lipid profiles of statin users with hyperlipidemia. These results are consistent with previous findings concerning the Chinese population9. In particular, the minor allele G carriers (GA genotype) of the SNP had higher HDL-C levels after 0–6 months (P = 0.013) and 7–12 months (P = 0.043) of statin treatment compared to the wild-type genotypes (Table 3). We believe that the difference in HDL-C levels between the GA genotypes is attributable to overexpression of the PSRC1 protein, which suppresses foam cell formation by upregulation of cholesterol transportation-related proteins in macrophages and the liver10.
Interestingly, females with the variant allele carriers of rs646776 showed higher HDL-C levels (P < 0.05) whereas males did not, suggesting a protective effect of the SNP against CHD and gene–gender interaction with the outcome of statin treatment. This supports our previous report that gender factors have a role in both statin efficacy and its muscular side effects11, 12. Apart from increased HDL-C levels in the blood, SORT1/CELSR2/PSRC1 rs646776 variants were associated with lower TC (P=0.007) and LDL-C (P=0.006) levels3. However, we were unable to relate the association of the rs646776 variants with statin-related TC and LDL-C lowering effects in the present study. Instead, the wild-type AA genotypes had significantly lower TC (P < 0.01) and LDL-C (P < 0.001) levels following statin treatment, although this was not observed in the GA genotypes (Table 3). The effect of the minor allele G on TC and LDL-C could be best described by quantile-dependent expressivity, which implies that rs646776 has an exclusive effect on statin efficacy13. The effect size of the SNP was determined by cholesterol levels, with smaller genetic effect sizes found at lower cholesterol levels (i.e., after statin treatment) or resulting in the opposite effect of the SNP on the percent of LDL-C change, particularly when cholesterol is decreased pharmacologically13. This explained the discrepancies in the SNP associations.
Furthermore, we were unable to link rs646776, the patient’s age, gender, and other clinical factors (the statin type and baseline lipid levels) to the patient’s attainment of an LDL-C level of < 2.6 mmol/L. This LDL-C level is the target for the 10-year prevention of CHD according to the National Cholesterol Education Program, Adult Treatment Panel III14. Higher baseline lipid values in patients before statin treatment did not influence their attainment of LDL-C < 2.6 mmol/L. rs646776 was identified as one of the two promising SNPs influencing statin-related lipid profiles in previous GWAS and multi-ethnic gene association studies. The influence of the abovementioned predicting variables on statin-affected lipid profiles is evident in this analysis as the inter-individual variability of lipid profiles is not solely determined by a single genetic polymorphism6, 15. As found in another study with a larger sample size (over 400,000 patients with Type 2 diabetes mellitus), it is possible that other patient factors, primarily their age and gender, correlate with the LDL-C profile16. With regards to the effect on LDL-C levels, a randomized controlled trial suggested that the type of statin had a significant impact on the LDL-C level17, implying that these independent factors were clinically meaningful when dealing with LDL-C profiles.
Since this is a preliminary investigation into the pharmacogenetic association between PSRC1/CELSR2/SORT1 rs646776 and statin-affected lipid profiles, the findings warrant replication with other statin-using Malaysian cohorts and different ethnicities from other Asian regions. Despite conflicting results regarding the extent to which SNP alters statin-affected lipid profiles4, 6, the rs646776 SNP, or its strongly linked rs599839 SNP, has been associated with the risk of CAD in White and Hispanic populations18, 19, 20, 21. The present study has limitations: the number of subjects in this study is relatively small compared to those in the abovementioned studies. Nevertheless, we demonstrated a clear association between PSRC1/CELSR2/SORT1 rs646776 and statin-affected lipid profiles, particularly HDL-C and LDL-C. Furthermore, the correlation between the studied genes and lipid profile changes was limited due to the absence of a homozygous recessive GG genotype. Larger sample sizes, typically between 400 and 500 subjects, are deemed sufficient to address research questions in gene association studies and increase the likelihood of capturing the GG genotype, as indicated by the reference population in Table 2. Last but not least, other confounding factors for lipid metabolism such as dietary intake, body mass index, and smoking status should be considered to ensure the internal validity of the results22, 23.
Conclusions
The present study found a significant association between the PSRC1/CELSR2/SORT1 rs646776 polymorphism and higher HDL-C levels among females taking statins. The findings warrant study replication with other Asian ethnicities.
Abbreviations
ACE: Angiotensin-converting enzyme, ANOVA: Analysis of variance, ARMS-PCR: Amplification refractory mutation system- polymerase chain reaction, BP: Base pair, DNA: Deoxyribonucleic acid, FH: Familial hypercholesterolemia, FI: Forward inner primer, FO: Forward outer primer, GWAS: Genome wide association study, HDL-C: High-density lipoprotein-cholesterol, HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A, HUSM: Hospital Universiti Sains Malaysia, HWE: Hardy-Weinberg equilibrium, LDL-C: Low-density lipoprotein-cholesterol, MAF: Minor allele frequencies, RI: Reverse inner primer, RO: Reverse outer primer, SEM: Standard error of the mean, SNP: Single nucleotide polymorphism, SORT1/CELSR2/PSRC1: Sortilin/cadherin EGF LAG seven-pass G-type receptor 2/proline-serine-rich coiled-coil protein 1, SPSS: Statistical package for the social sciences, TC: Total cholesterol, TG: Triglycerides
Acknowledgments
None
Author’s contributions
Conceptualization, N.S.B. and Z.Z.; methodology, N.S.B., A.F.S. and Z.Z.; software, R.Z. and A.F.S; validation, R.Z. and N.S.B.; formal analysis, R.Z. and N.S.B.; investigation, R.Z. and A.F.S.; resources, N.S.B. and Z.Z.; data curation, R.Z., A.F.S. and N.S.B.; writing - original draft preparation, R.Z. and N.S.B., writing- review and editing, R.Z. and N.S.B.; visualization, R.Z.; supervision, N.S.B. and Z.Z.; project administration, N.S.B.; funding acquisition, N.S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Ministry of Higher Education Malaysia for Fundamental Research Grant Scheme with Project Code: FRGS/1/2022/SKK10/USM/03/10.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the USM Human Research Ethics Committee (Approval number: USM/JePeM/19070437). Informed consent was obtained from all patients involved in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
-
Coronary Artery Disease (C4D) Genetics Consortium
A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nature Genetics.
2011;
43
(4)
:
339-44
.
View Article PubMed Google Scholar -
Musunuru
K.,
Strong
A.,
Frank-Kamenetsky
M.,
Lee
N.E.,
Ahfeldt
T.,
Sachs
K.V.,
From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature.
2010;
466
(7307)
:
714-9
.
View Article PubMed Google Scholar -
Arvind
P.,
Nair
J.,
Jambunathan
S.,
Kakkar
V.V.,
Shanker
J.,
CELSR2-PSRC1-SORT1 gene expression and association with coronary artery disease and plasma lipid levels in an Asian Indian cohort. Journal of Cardiology.
2014;
64
(5)
:
339-46
.
View Article PubMed Google Scholar -
Hopewell
J.C.,
Parish
S.,
Offer
A.,
Link
E.,
Clarke
R.,
Lathrop
M.,
Heart Protection Study Collaborative Group
MRC/BHF,
Impact of common genetic variation on response to simvastatin therapy among 18 705 participants in the Heart Protection Study. European Heart Journal.
2013;
34
(13)
:
982-92
.
View Article PubMed Google Scholar -
Strong
A.,
Rader
D.J.,
Sortilin as a regulator of lipoprotein metabolism. Current Atherosclerosis Reports.
2012;
14
(3)
:
211-8
.
View Article PubMed Google Scholar -
Postmus
I.,
Trompet
S.,
Deshmukh
H.A.,
Barnes
M.R.,
Li
X.,
Warren
H.R.,
Welcome Trust Case Control Consortium
Pharmacogenetic meta-analysis of genome-wide association studies of LDL cholesterol response to statins. Nature Communications.
2014;
5
(1)
:
5068
.
View Article PubMed Google Scholar -
Peters
B.J.,
Pett
H.,
Klungel
O.H.,
Stricker
B.H.,
Psaty
B.M.,
Glazer
N.L.,
Genetic variability within the cholesterol lowering pathway and the effectiveness of statins in reducing the risk of MI. Atherosclerosis.
2011;
217
(2)
:
458-64
.
View Article PubMed Google Scholar -
Patnaik
S.,
Pollevick
M.E.,
Lara-Breitinger
K.M.,
Stone
N.J.,
Inter-Individual Variability in Lipid Response: A Narrative Review. The American Journal of Medicine.
2022;
135
(12)
.
View Article PubMed Google Scholar -
Zhou
Y.J.,
Hong
S.C.,
Yang
Q.,
Yin
R.X.,
Cao
X.L.,
Chen
W.X.,
Association of variants in CELSR2-PSRC1-SORT1 with risk of serum lipid traits, coronary artery disease and ischemic stroke. International Journal of Clinical and Experimental Pathology.
2015;
8
(8)
:
9543-51
.
PubMed Google Scholar -
Guo
K.,
Hu
L.,
Xi
D.,
Zhao
J.,
Liu
J.,
Luo
T.,
PSRC1 overexpression attenuates atherosclerosis progression in apoE-/- mice by modulating cholesterol transportation and inflammation. Journal of Molecular and Cellular Cardiology.
2018;
116
:
69-80
.
View Article PubMed Google Scholar -
Bakar
N.S.,
Neely
D.,
Avery
P.,
Brown
C.,
Daly
A.K.,
Kamali
F.,
Genetic and Clinical Factors Are Associated With Statin-Related Myotoxicity of Moderate Severity: A Case-Control Study. Clinical Pharmacology and Therapeutics.
2018;
104
(1)
:
178-87
.
View Article PubMed Google Scholar -
Shamsudin
A.F.,
Bakar
N.S.,
Gender Differences in the Association between Cholesteryl Esters Transfer Protein Polymorphism (rs708272) and Plasma Lipid Levels in Hyperlipidaemic Participants at Hospital Universiti Sains Malaysia. The Malaysian Journal of Medical Sciences : MJMS.
2023;
30
(2)
:
96-110
.
View Article PubMed Google Scholar -
Williams
P.T.,
Quantile-specific heritability of total cholesterol and its pharmacogenetic and nutrigenetic implications. International Journal of Cardiology.
2021;
327
:
185-92
.
View Article PubMed Google Scholar -
Safeer
R.S.,
Ugalat
P.S.,
Cholesterol treatment guidelines update. American Family Physician.
2002;
65
(5)
:
871-80
.
PubMed Google Scholar -
Lanktree
M.B.,
Anand
S.S.,
Yusuf
S.,
Hegele
R.A.,
Investigators
SHARE,
Replication of genetic associations with plasma lipoprotein traits in a multiethnic sample. Journal of Lipid Research.
2009;
50
(7)
:
1487-96
.
View Article PubMed Google Scholar -
Russo
G.,
Pintaudi
B.,
Giorda
C.,
Lucisano
G.,
Nicolucci
A.,
Cristofaro
M.R.,
Age- and gender-related differences in LDL-cholesterol management in outpatients with type 2 diabetes mellitus. International Journal of Endocrinology.
2015;
2015
:
957105
.
View Article PubMed Google Scholar -
Welty
F.K.,
Lewis
S.J.,
Friday
K.E.,
Cain
V.A.,
Anzalone
D.A.,
A Comparison of Statin Therapies in Hypercholesterolemia in Women: A Subgroup Analysis of the STELLAR Study. Journal of Women's Health.
2016;
25
(1)
:
50-6
.
View Article PubMed Google Scholar -
Kleber
M.E.,
Renner
W.,
Grammer
T.B.,
Linsel-Nitschke
P.,
Boehm
B.O.,
Winkelmann
B.R.,
Association of the single nucleotide polymorphism rs599839 in the vicinity of the sortilin 1 gene with LDL and triglyceride metabolism, coronary heart disease and myocardial infarction. The Ludwigshafen Risk and Cardiovascular Health Study. Atherosclerosis.
2010;
209
(2)
:
492-7
.
View Article PubMed Google Scholar -
Lee
J.Y.,
Lee
B.S.,
Shin
D.J.,
Woo Park
K.,
Shin
Y.A.,
Joong Kim
K.,
A genome-wide association study of a coronary artery disease risk variant. Journal of Human Genetics.
2013;
58
(3)
:
120-6
.
View Article PubMed Google Scholar -
Angelakopoulou
A.,
Shah
T.,
Sofat
R.,
Shah
S.,
Berry
D.J.,
Cooper
J.,
Comparative analysis of genome-wide association studies signals for lipids, diabetes, and coronary heart disease: Cardiovascular Biomarker Genetics Collaboration. European Heart Journal.
2012;
33
(3)
:
393-407
.
View Article PubMed Google Scholar -
Qi
L.,
Ma
J.,
Qi
Q.,
Hartiala
J.,
Allayee
H.,
Campos
H.,
Genetic risk score and risk of myocardial infarction in Hispanics. Circulation.
2011;
123
(4)
:
374-80
.
View Article PubMed Google Scholar -
Serdar
C.C.,
Cihan
M.,
Yücel
D.,
Serdar
M.A.,
Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb).
2021;
31
(1)
:
010502
.
View Article PubMed Google Scholar -
Skelly
A.C.,
Dettori
J.R.,
Brodt
E.D.,
Assessing bias: the importance of considering confounding. Evidence-Based Spine-Care Journal.
2012;
3
(1)
:
9-12
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 10 No 12 (2023)
Page No.: 6110-6117
Published on: 2023-12-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 3670 times
- PDF downloaded - 1251 times
- Appendix downloaded - 951 times
- XML downloaded - 103 times
 Biomedpress
Biomedpress



